
| A��NaClO��Һ��FeCl2��Һ��ϣ�6Fe2����3ClO����3H2O=2Fe(OH)3����3Cl����4Fe3�� |
| B����ʳ���������е�̼��ƣ�CaCO3��2H��=Ca2����CO2����H2O |
| C��FeCl2������Һ���ڿ����б��ʣ�2Fe2����4H����O2=2Fe3����2H2O |
D�����MgCl2ˮ��Һ�����ӷ���ʽ��2Cl����2H2O H2����Cl2����2OH�� H2����Cl2����2OH�� |
 Mg(OH)2����H2����Cl2����
Mg(OH)2����H2����Cl2����


| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ������ ���ͣ������

| ���� | ���Ӱ뾶(pm) | ��ʼ���� ʱ��pH | ��ȫ���� ʱ��pH |
| Fe2�� | 74 | 7.6 | 9.7 |
| Fe3�� | 64 | 2.7 | 3.7 |
| Al3�� | 50 | 3.8 | 4.7 |
| Mn2�� | 80 | 8.3 | 9.8 |
| Pb2�� | 121 | 8.0 | 8.8 |
| Ca2�� | 99 | �� | �� |

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
A���Ȼ�����Һ�м��������ˮ��Al3����4NH3��H2O=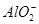 ��4 ��4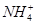 |
B��ͭƬ�ӵ�Դ������̼���ӵ�Դ���������������Һ�� |
C������һ������Һˮ�⣺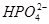 ��H2O ��H2O 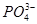 ��H3O�� ��H3O�� |
| D��ʵ�������Ƶ���������Һ�ڿ����б�������4Fe2����O2��2H2O=4Fe3����4OH�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A����Mg(HCO3)2��Һ�м��������NaOH��Һ��Mg2����2HCO3����4OH��=Mg(OH)2����2CO32����2H2O |
| B����NH4Al(SO4)2��Һ�е���Ba(OH)2��Һǡ��ʹSO42����Ӧ��ȫ��2Ba2����4OH����Al3����2SO42��=2BaSO4����AlO2����2H2O |
| C��FeBr2��Һ��ͨ�������Cl2��2Fe2����4Br����3Cl2=2Fe3����2Br2��6Cl�� |
| D����Fe(NO3)2��Һ�м���ϡ���3Fe2����4H����NO3��=3Fe3����NO����2H2O |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A�����������Һ��ͨ�������������Ca2+ + 2ClO��+ H2O + SO2��CaSO3��+ 2HClO |
| B���������������һ��ʱ����Һ��pH�½���2H2SO3��O2��2H2SO4 |
C������Һ������ϴ���۵�ԭ��CO32¯+H2O HCO3¯+OH¯ HCO3¯+OH¯ |
D����K2Cr2O7��Һ�м�������NaOHŨ��Һ����Һ�ɳ�ɫ��Ϊ��ɫ��Cr2O72��+H2O 2CrO42��+2H+ 2CrO42��+2H+ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A��Na2O2����ˮ����O2��2O22����2H2O=O2����4OH�� |
| B����ˮͨ������SO2��SO2��2NH3��H2O=2NH4����SO32����H2O |
| C������������Ũ���ᷴӦ����Cl2��ClO����Cl����H2O=Cl2����2OH�� |
| D��NaHCO3��Һ������Ba(OH)2��Һ��Ӧ��Ba2����2OH����2HCO3��=BaCO3����CO32����2H2O |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
A������������������ˮ��Һ�еķ�Ӧ��CH3CH2Br + OH-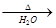 CH3CH2OH +Br- CH3CH2OH +Br- |
B��������Һ��������ͭ��Ӧ��2CH3COOH + Cu(OH)2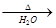 Cu2+ + 2CH3COO-+ 2H2O Cu2+ + 2CH3COO-+ 2H2O |
C����������Һ��ͨ������������̼��2C6H5O��+ CO2 + H2O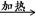 2C6H5OH + CO32- 2C6H5OH + CO32- |
D����ȩ��Һ��������������Һ����HCHO+4[Ag(NH3)2]++4OH��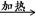 CO32-+2NH4+ +4Ag��+6NH3+2H2O CO32-+2NH4+ +4Ag��+6NH3+2H2O |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A��������Һ�м�������������þ���壺Mg(OH)2+2CH3COOH=Mg2++2CH3COO-+2H2O |
| B��H2O2��Һ�м�����������KMnO4��Һ��2MnO4-+3H2O2+6H+=2Mn2++6H2O+4O2�� |
| C��Ca(HCO3)2��Һ�м�����������ʯ��ˮ��Ca2++HCO3-+OH-=CaCO3��+H2O |
| D��NH4HSO4��ϡ��Һ����μ���Ba(OH)2��Һ��SO42��ǡ�ó�����ȫ��NH4++H++SO42-+Ba2++2OH-=NH3?H2O+BaSO4��+H2O |
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com