
����98��(�ܶ�Ϊ1.84 g��cm��3)��Ũ��������Ũ��Ϊ0.25 mol��L��1��ϡ����1000 mL����Ҫ�ش��������⣺
(1)��ҪȡŨ��������________mL
(2)���ʵ������15 mL��20 mL��50 mL����ͲӦѡ��________mL����Ͳ��ã���ȡʱ������Ͳ���ɾ���ˮϴ����ֱ����ȡ��ʹ���_______��(�ƫ�ߡ���ƫ�͡�����Ӱ�족)
(3)��Ũ�������ձ��ڱ�����ע��ʢˮ���ձ��У������Ͻ����Ŀ����_______���������������Һ�彦���������ʹŨ��ƫ_______��
(4)��ת������ƿǰ�ձ���Һ��Ӧ_______�������ʹ���Ũ��ƫ_______����ϴ���ձ�2��3�Σ�ϴ��ҺҲҪת������ƿ�������ʹ���Ũ��_______��
(5)����ʱ����ʹ��Һ��Һ����̶���ƽ�������ӻ�ʹ���Ũ��_______��
(6)���Ʋ����ɷֽ�����¼�����������ȷ�IJ���˳����_______��
A��������ƿ��ע����������ˮ������Ƿ�©ˮ
B������������ˮϴ���ձ���������������Һע������ƿ�����ظ���������
C��������ȴ��ϡ����ע���Ѽ�鲻©ˮ������ƿ��
D�����ݼ��㣬����Ͳ��ȡһ�������Ũ����
E����Ũ�������ձ�������ע��ʢ������ˮ��С�ձ��У��������ò���������
F����������ƿ���ӣ���ҡ��
G���ý�ͷ�ιܵμ�����ˮ��ʹ��Һ����ǡ����̶�����
H������������ƿ��С�ĵؼ�����ˮ��ʹҺ��ӽ��̶���

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 ������������Ϊ98%���ܶ�Ϊ1.84g/cm3��Ũ���ᣬ����0.2mol/L��������Һ480mL���Իش��������⣺
������������Ϊ98%���ܶ�Ϊ1.84g/cm3��Ũ���ᣬ����0.2mol/L��������Һ480mL���Իش��������⣺�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
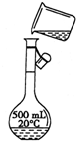 ������������Ϊ98%���ܶ�Ϊ1.84g/cm3��Ũ���ᣬ����0.2mol/L��������Һ500mL���Իش��������⣺
������������Ϊ98%���ܶ�Ϊ1.84g/cm3��Ũ���ᣬ����0.2mol/L��������Һ500mL���Իش��������⣺�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com