
���֣�����A��E��ת����ϵ��ͼ��ʾ:
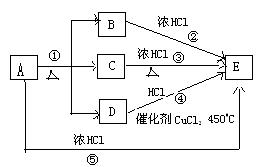
��֪���嵥��D��ʹ�����ǵ�ľ����ȼ,��Ӧ����ʵ�����Ƶ���D�ij��÷���֮һ,��Ӧ����ʵ�����ƻ���ɫ����E����Ҫ��Ӧ,(����������δ�г�)��
����������Ϣ,�ش��������⣺
(1)A�Ļ�ѧʽ
(2) ����Ԫ�ػ��ϼ۵ı仯��������д����Ӧ�ڢۢܢ����ַ����Ĺ�ͬ����
(3)д����Ӧ�ܵĻ�ѧ����ʽ
(4)���÷�Ӧ�ۢܢݵı仯����д��ѧ����ʽ�Ƚ�A��C��D���������ɴ�С��˳��
(5)д����Ӧ�ڵ����ӷ���ʽ ,�������뻹ԭ�������ʵ���֮��
��1��KMnO4
��2����Ԫ�صĻ��ϼ���-1�����ߵ�0��
��3��4HCl+O2![]() 2Cl2+2H2O
2Cl2+2H2O
��4��KMnO4>MnO2>O2
(5) MnO2��4H++2Cl-![]() Mn2++Cl2��+2H2O�� 1:2
Mn2++Cl2��+2H2O�� 1:2
������������������ʵ�����Ʊ�Ϊ�زģ�ע�������Ʊ�������Ϊ������ԭ��Ӧ�����߶��Ը������Ϊ��Ӧ����ǽ����ͻ�ƿڡ�����Ȼ������ɫ���嵥��Ϊ����������DΪ������AΪ������ء���ͼ���յ�����Ϊ���������Ǻ����ᷴӦ�����ԣ��ڢۢܢ��ĸ���Ӧ�Ĺ�ͬ��Ϊ�ò�ͬ������������Ũ��������Ϊ��������Ԫ�صĻ��ϼ���-1�����ߵ�0�ۡ����ݢۢܢ�������Ӧ�з�Ӧ�����IJ��죨���·�Ӧ�����ȡ����ȴ��������ó�A��C��D���������ɴ�С��˳��ΪKMnO4>MnO2>O2��



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
(12��)A��G�����ʼ�Ĺ�ϵ����ͼ������B��DΪ��̬���ʡ�
MnO2��Fe��ȼ
MnO2�ڡ�
��ش��������⣺
(1)����C��E�����Ʒֱ�Ϊ________��________��
(2)��ѡ�ò�ͬ��A���з�Ӧ�٣������ڳ����½��У��仯ѧ����ʽ___________��
��ֻ���ڼ�������½��У���Ӧ��AӦΪ________��
(3)��Ӧ�ڵĻ�ѧ����ʽΪ_____________________��
(4)�����Ƶ�F��ҺӦ����________�Է�ֹ��ת��ΪG������G��Һ�������ӵij����Լ�
��________��ʵ������Ϊ_____________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
(10��)A��G�����ʼ�Ĺ�ϵ����ͼ������B��DΪ��̬���ʡ�
��ش��������⣺
(1)����C��E�����Ʒֱ�Ϊ________________��__________________��
(2)��ѡ�ò�ͬ��A���з�Ӧ�٣������ڳ����½��У��仯ѧ����ʽΪ_____________��
��ֻ���ڼ�������½��У���Ӧ��AӦΪ_____________��
(3)��Ӧ�ڵĻ�ѧ����ʽΪ_______________________________________��
(4)�����Ƶ�F��ҺӦ����_____________�Է�ֹ��ת��ΪG������G��Һ�������ӵij����Լ���_____________��ʵ������Ϊ_____________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011-2012ѧ�꺣��ʡ�λ���ѧ��һ��ѧ�ڽ�ѧ�����������ѧ�Ծ� ���ͣ������
(12��)A��G�����ʼ�Ĺ�ϵ����ͼ������B��DΪ��̬���ʡ�
MnO2��Fe��ȼ
MnO2�ڡ�
��ش��������⣺
(1)����C��E�����Ʒֱ�Ϊ________��________��
(2)��ѡ�ò�ͬ��A���з�Ӧ�٣������ڳ����½��У��仯ѧ����ʽ___________��
��ֻ���ڼ�������½��У���Ӧ��AӦΪ________��
(3)��Ӧ�ڵĻ�ѧ����ʽΪ_____________________��
(4)�����Ƶ�F��ҺӦ����________�Է�ֹ��ת��ΪG������G��Һ�������ӵij����Լ�
��________��ʵ������Ϊ_____________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2010��߿���ѧ�������ר��ʮһ ����Ԫ�ؼ��仯���� ���ͣ������
(8��)A��G�����ʼ�Ĺ�ϵ����ͼ������B��DΪ��̬���ʡ�
��ش��������⣺
(1)����C��E�����Ʒֱ�Ϊ________________��__________________��
(2)��ѡ�ò�ͬ��A���з�Ӧ�٣������ڳ����½��У��仯ѧ����ʽΪ_____________��
��ֻ���ڼ�������½��У���Ӧ��AӦΪ_____________��
(3)��Ӧ�ڵĻ�ѧ����ʽΪ_______________________________________��
(4)�����Ƶ�F��ҺӦ����_____________�Է�ֹ��ת��ΪG������G��Һ�������ӵij����Լ���_____________��ʵ������Ϊ______________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2010����ͨ�ߵ�ѧУ����ȫ��ͳһ���Ի�ѧ���⣨���Ͼ��� ���ͣ������
(8��)A��G�����ʼ�Ĺ�ϵ����ͼ������B��DΪ��̬���ʡ�

��ش��������⣺
(1)����C��E�����Ʒֱ�Ϊ________________��__________________��
(2)��ѡ�ò�ͬ��A���з�Ӧ�٣������ڳ����½��У��仯ѧ����ʽΪ_____________��
��ֻ���ڼ�������½��У���Ӧ��AӦΪ_____________��
(3)��Ӧ�ڵĻ�ѧ����ʽΪ_______________________________________��
(4)�����Ƶ�F��ҺӦ����_____________�Է�ֹ��ת��ΪG������G��Һ�������ӵij����Լ���_____________��ʵ������Ϊ______________________________________��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com