
19-I�����йؾ���ṹ�����ʵ���������ȷ���ǣ�______��
A.���д��ڼ��Լ������Ӽ������������
B.�������K>Na���ʽ����ص��۵���ڽ�����
C.��1mol�Ľ��ʯ��ʯī������������C-C������Ŀ��ͬ
D.����þ�ľ����ܴ����Ȼ��ƣ������۵�����Ȼ��ơ�
19-��ij������Ͻ�Ҳ��Ϊ������������磺Cu9Al4��Cu5Zn8�ȡ����ʴ��������⣺

(1)��̬пԭ�ӵĵ����Ų�ʽΪ_______________________________����֪����п������Ũ���ռ���Һ���ɿ����Ե����ǻ���п����Na2[Zn(OH)4]���������÷�Ӧ�����ӷ���ʽΪ�� ___________________________________________________����֪���ǻ���п�����ӿռ乹�������������ͣ���Zn2+���ӻ���ʽΪ__________________��
��2��ͭ����±��(SCN)2��Ӧ������Cu(SCN)2,1mol (SCN)2�����к���__________���Ҽ�����±��(SCN)2��Ӧ���������֣�A��������(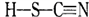 )��B-��������(
)��B-��������(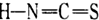 )������Ϊ��_________�������۵�ϸߵ���___________ (�����)��ԭ����________________________________��
)������Ϊ��_________�������۵�ϸߵ���___________ (�����)��ԭ����________________________________��
��3����֪��п������ͼ1��ʾ��������Zn2+����λ����____________�� S2-��ȡ�Ķѻ���ʽΪ____________________������A1��A2��A3��
��4����֪ͭ����γɵĽ���������Ľṹ��ͼ2��ʾ���������������ⳤΪa����(nm)���ý�����������ܶ�Ϊ_______g/cm3���ú�a��NA�Ĵ���ʽ��ʾ����
 ���ƽ̸�������ѡ����ĩ���100��ϵ�д�
���ƽ̸�������ѡ����ĩ���100��ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ��2016-2017ѧ��ӱ�ʡ�߶���ѧ�ڵ�һ���¿���ѧ�Ծ��������棩 ���ͣ�ѡ����
���г�ȥ���ʵķ�����ȷ���ǣ� ��
�ٳ�ȥ��������������ϩ������������ͨ��Cl2����Һ����
�ڳ�ȥ�����Ҵ�������������ñ���̼������Һϴ�ӡ���Һ
�۳�ȥCO2��������SO2������ͨ��ʢ�б���̼��������Һ��ϴ��ƿ
�ܳ�ȥ�Ҵ��������������������ʯ�ң�����
A. �٢� B. �ۢ� C. �ڢۢ� D. �ڢ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2017������и�����ѧ�ڵ�һ���¿���ѧ�Ծ��������棩 ���ͣ��ƶ���
�л���M���л��ϳɵ���Ҫ�м��壬�Ʊ�M��һ�ֺϳ�·�����£����ַ�Ӧ�������Լ�����ȥ��
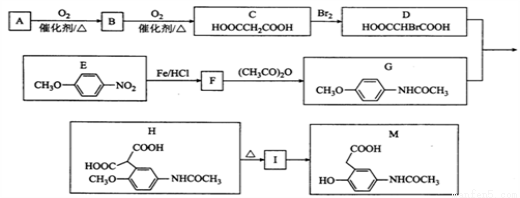
��֪�� ��A���ܶ�����ͬ������H2�ܶȵ�38��������ӵĺ˴Ź�����������3��壻
��
��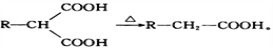
��ش��������⣺
��1��B�Ļ�ѧ����Ϊ______________��A�й����ŵĵ���ʽΪ________________��
��2��C�й����ԭ�������________����I�Ľṹ��ʽΪ_____________________��
��3��F��G�Ļ�ѧ����ʽΪ________________________________________________��
��4��M�����ܷ����ķ�ӦΪ_______________����ѡ����ĸ��
A.�ӳɷ�Ӧ B.������Ӧ C.ȡ����Ӧ D.��ȥ��Ӧ
��5��ͬʱ��������������E��ͬ���칹����_________�֡�
������FeCl3��Һ������ɫ��Ӧ ������NaHCO3��Ӧ �ۺ��С�����NH2
��6�����������ϳ�·�ߣ��� 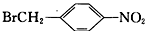 Ϊԭ�ϣ����Լ���ѡ��������Ʊ�
Ϊԭ�ϣ����Լ���ѡ��������Ʊ�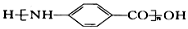 �ĺϳ�·�ߣ�_______________________________________________��
�ĺϳ�·�ߣ�_______________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2016-2017ѧ��ɽ��ʡ��һ3���¿���ѧ�Ծ��������棩 ���ͣ�ѡ����
��ͼ��Ԫ�����ڱ���һ����,��֪������Ӱ�м��3������ͬһ�塣�й���Ӱ���ֵ�Ԫ��,����˵����ȷ����(����)

A. ��������Ԫ�� B. ���Ǹ���Ԫ��
C. ��5�ָ���Ԫ�غ�2������Ԫ�� D. ��5������Ԫ�غ�2�ָ���Ԫ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2016-2017ѧ��ɽ��ʡ��һ3���¿���ѧ�Ծ��������棩 ���ͣ�ѡ����
����������Ԫ��X��Y��Z��W��ԭ��������������Xԭ�ӵ����������������ڲ��������2��,Y�ǵؿ��к�����ߵ�Ԫ��,Z2+��Y2-������ͬ�ĵ��Ӳ�ṹ,W��Xͬ���塣����˵����ȷ����(����)
A. Y����̬���⻯������ȶ��Ա�W��ǿ
B. Y�ֱ���Z��W�γɵĻ������л�ѧ��������ͬ
C. X������������Ӧ��ˮ��������Ա�W����
D. ԭ�Ӱ뾶�Ĵ�С˳��:r(W)>r(Z)>r(Y)>r(X)
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ʡ���IJ���ѧ2017�������ѧ��������ѧ�Ծ� ���ͣ������
������(CH3OCH3)����Ϊ21���͵�����ȼ�ϣ���CO��H2Ϊԭ��������������Ҫ��������������Ӧ��
��ѧ��Ӧ����ʽ | ��ѧƽ�ⳣ�� | |
��CO(g)��2H2(g) | ��H1=-99 kJ•mol-1 | K1 |
��2CH3OH(g) | ��H2����24 kJ•mol-1 | K2 |
��CO(g)��H2O(g) | ��H3����41 kJ•mol-1 | K3 |
��1���ù��յ��ܷ�ӦΪ3CO(g)��3H2(g) CH3OCH3(g)��CO2(g) ��H
CH3OCH3(g)��CO2(g) ��H
�÷�Ӧ��H��__________________����ѧƽ�ⳣ��K��____________________(�ú�K1��K2��K3�Ĵ���ʽ��ʾ)��
��2��ij�¶��£���8.0molH2��4.0molCO�����ݻ�Ϊ2L���ܱ������У�������Ӧ��4H2(g)+2CO(g)  CH3OCH3(g)+H2O(g)��10 ���Ӻ�Ӧ��ƽ�⣬��ö����ѵ��������Ϊ25%����CO��ת����Ϊ________��
CH3OCH3(g)+H2O(g)��10 ���Ӻ�Ӧ��ƽ�⣬��ö����ѵ��������Ϊ25%����CO��ת����Ϊ________��
��3�����д�ʩ�У������CH3OCH3���ʵ���________��
A������������� B�������¶� C�����ø�Ч���� D������ѹǿ
��4���ù����з�Ӧ�۵ķ��������CH3OCH3�IJ��ʣ�ԭ����_______________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ʡ���IJ���ѧ2017�������ѧ��������ѧ�Ծ� ���ͣ�ѡ����
����ʵ��������KMnO4��Һ�ܴﵽԤ��Ŀ����
A. ���eSO2��CO2 B. ���𱽺ͼױ�
C. ����CH2=C (CH3) CH2OH�к�̼̼˫�� D. ��������������Һ���Ƿ���������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2016-2017ѧ���Ĵ�ʡ�ɶ��б���У���߶�3���¿���ѧ�Ծ��������棩 ���ͣ�ѡ����
374�桢22.1Mpa���ϵij��ٽ�ˮ���к�ǿ���ܽ��л���������������н϶��H+��OH�C���ɴ˿�֪���ٽ�ˮ
A�������ԣ�pH����7 B�����ֳ��Ǽ����ܼ�������
C�������ԣ�pHС��7 D�����ֳ������ܼ�������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2017���㽭ʡ����������ȫ������3��������ѡ����Ŀ����ѧ�Ծ��������棩 ���ͣ�ʵ����
��Zn����Ҫ����Fe��Al��Pb���ʣ�����������ȡH2�����������Һ�Ʊ�����п����(ZnSO4��7H2O)��Al2O3��Fe2O3���������£�
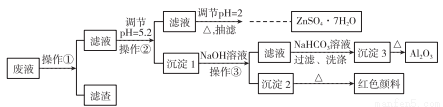
��֪Al3+��Fe3+��Zn2+������������ȫ������pH�ֱ�Ϊ5.2��4.1��8.5��ZnSO4��7H2O����������ˮ���绯���ش��������⣺
(1)����pH=2��Ŀ����______________������pH=2���ɼ���_________���ѧʽ����
(2)д�����ɳ���3�Ļ�ѧ����ʽ��______________________��
(3)����Ũ��ZnSO4��Һ���ּ���ƷĤʱ��Ҫֹͣ���ȵ���Ҫԭ����_____________��
(4)ijͬѧ����ͼ��ʾ��װ�ó��ˡ�
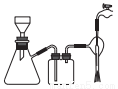
���йس��˵�˵����ȷ����__________��
A.���˵�Ŀ����Ҫ�ǵõ��ϸ���ij���
B����ֽ��ֱ��Ӧ��С��©���ھ������ܸ�סȫ��С��
C��ͼ����һ������
D�����˽�����������ƿ��֧�ܿڵ�����Һ
�ڳ��ˣ�ϴ�ӳ����ľ��������___________________________��
(5)Ϊ�õ������ZnSO4��7H2O��Ʒ��ѡ����﷽����________��
A.���Ⱥ�� B����ŨH2SO4����
C���þƾ�ϴ�� D���ڿ����а�Ȼ����
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com