
�л���M���л��ϳɵ���Ҫ�м��壬�Ʊ�M��һ�ֺϳ�·�����£����ַ�Ӧ�������Լ�����ȥ��
��֪�� ��A���ܶ�����ͬ������H2�ܶȵ�38��������ӵĺ˴Ź�����������3��壻
��
��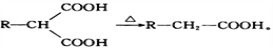
��ش��������⣺
��1��B�Ļ�ѧ����Ϊ______________��A�й����ŵĵ���ʽΪ________________��
��2��C�й����ԭ�������________����I�Ľṹ��ʽΪ_____________________��
��3��F��G�Ļ�ѧ����ʽΪ________________________________________________��
��4��M�����ܷ����ķ�ӦΪ_______________����ѡ����ĸ��
A.�ӳɷ�Ӧ B.������Ӧ C.ȡ����Ӧ D.��ȥ��Ӧ
��5��ͬʱ��������������E��ͬ���칹����_________�֡�
������FeCl3��Һ������ɫ��Ӧ ������NaHCO3��Ӧ �ۺ��С�����NH2
��6�����������ϳ�·�ߣ��� 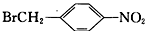 Ϊԭ�ϣ����Լ���ѡ��������Ʊ�
Ϊԭ�ϣ����Լ���ѡ��������Ʊ�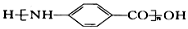 �ĺϳ�·�ߣ�_______________________________________________��
�ĺϳ�·�ߣ�_______________________________________________��
 ������������Ӧ����ϵ�д�
������������Ӧ����ϵ�д� ͬ����չ�Ķ�ϵ�д�
ͬ����չ�Ķ�ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
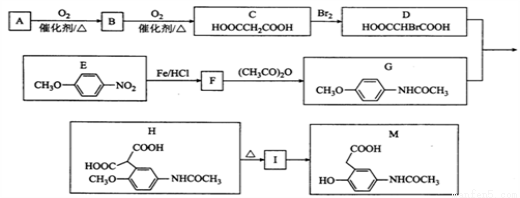
��Ŀ�����л�ѧ ��Դ��2017�����ʡ�����и�����ѧ�ڵ�������Ӧ�Կ������ۻ�ѧ�Ծ��������棩 ���ͣ������
2015��10�£��й���ѧ�������ϻ����2015���ŵ��������ѧ��ҽѧ�����������ǡ���Ϊ���������ء���һ����������ű����ҩ������ȫ���e�Ƿ�չ�й��ҵ��������˵�����������֪�����ص�һ�ֻ�ѧ�ϳɷ����IJ��ֹ���������ͼ24��
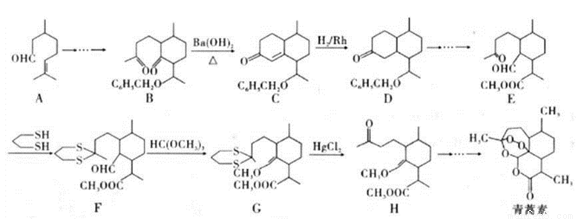
��֪����
��
��մ��������⣺
(1)�����й�˵����ȷ����_______(�����)��
A.���������ڻ�״������������ڷ����廯����
B.������������ˮ���������Ҵ�������
C.һ�������£���������������������Һ��Ӧ
D.��������ʪ��ĵ��۵⻯����ֽ��������ɫ������Ϊ���ӽṹ�к�������
(2)������A�к��еķǺ��������ŵ�������_______����ѡ�����к��ʵ��Լ�������ù����ţ��Լ��������ȷ˳��Ϊ____��
A.��ˮ B.ϡ����
C.����������ͭ����Һ D.����������Һ
(3)�ù������������E��F��G��H��Ŀ����_________________��
(4)H��ϡ���Ṳ��ʱ��Ӧ�Ļ�ѧ����ʽΪ_____________________��
(5)M��A��Ϊͬϵ�����A������̼ԭ�ӡ���������������M��ͬ���칹����______�֣������������칹����
�ٺ�����Ԫ�� ���ܷ���������Ӧ
(6)������ѧ֪ʶ��������Ϣ��д���Ա���ȩ��������Ϊԭ�ϣ��Ʊ��л���ȩ�� CH2CH2CHO)��·������ͼ��_______________________�����Լ���ѡ���ϳ�·������ͼʾ�����£�
CH2CH2CHO)��·������ͼ��_______________________�����Լ���ѡ���ϳ�·������ͼʾ�����£�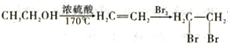 ��
��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2017�����ʡ��ɳ�и�����ʵ��ࣩ��ʮ���¿����ۻ�ѧ�Ծ��������棩 ���ͣ�ѡ����
���й����л����������ȷ����
A. ��ϩ��������ϩ�ͱ������о�����̼̼˫��
B. ������֬������ʹ����KMnO4��Һ��ɫ
C. �ȱ�����������ԭ�Ӷ�����ͬһƽ��
D. �ױ������ϵ�һ����ԭ�ӱ���C3H6Clȡ�����γɵ�ͬ���칹����9��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2016-2017ѧ�����ʡ�߶�3���¿���ѧ�Ծ��������棩 ���ͣ�ѡ����
�����й��л�������ṹ�����ʵ�˵����ȷ����
A. ��ϩ������������������������ӳɷ�Ӧ
B. ij�������ķ���ʽΪC10H14������������ʹ����KMnO4��Һ��ɫ���ҷ��ӽṹ��ֻ��һ����������������������4��
C. 1 mol���л���ṹ����ͼ��������뺬5mol NaOH��ˮ��Һ��ȫ��Ӧ
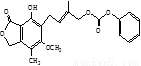
D. ������������ʹ������ʧȥ�������ԣ����ȡ�����������ȴ�ʩ��ʹ�����ʱ���
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2016-2017ѧ�����ʡ�߶�3���¿���ѧ�Ծ��������棩 ���ͣ�ѡ����
���й����л�������Ľṹ�����ʵ�������ȷ���ǣ� ��
A. ���ࡢ��֬�������ʵ�ˮ����ﶼ�Ƿǵ����
B. ���ۺ���ά�صķ���ʽ��Ϊ��C6H10O5��n������Ϊͬ���칹��
C. ��ά�ء����ͷֱ���Ũ�������ʱ��Ũ����ķ�Ӧ����ͬһ���͵ķ�Ӧ
D. �Ҵ�������ͱ��ӵķ����о����й����š�OH�����Ծ�����NaOH��Һ��Ӧ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2017������и�����ѧ�ڵ�һ���¿���ѧ�Ծ��������棩 ���ͣ�ѡ����
����ʵ�鷽���У����ܴﵽʵ��Ŀ�ĵ���
ѡ�� | ʵ��Ŀ�� | ʵ�鷽�� |
A | ����CH3CH2Br��NaOH��Һ���Ƿ���ˮ�� | ��CH3CH2Br��NaOH��Һ���ȡ���ȴ��ȡ���ϲ�ˮ��Һ����ϡHNO3�ữ������AgNO3��Һ���۲��Ƿ��������ɫ���� |
B | ����Fe(NO3)2�����Ƿ����������� | ��Fe(NO3)2��Ʒ����ϡH2SO4�μ�KSCN��Һ���۲���Һ�Ƿ��� |
C | ��֤Br2��������ǿ��I2 | ��������ˮ����KI��Һ�У��ټ���CCl4�������ã��ɹ۲쵽�²�Һ�����ɫ |
D | ��֤AgI���ܽ��С��AgCl | ��NaIŨ��Һ����AgCl����Һ�У����ɹ۲쵽�����ɰ�ɫ��Ϊ��ɫ |
A. A B. B C. C D. D
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2016-2017ѧ��ɽ��ʡ��һ3���¿���ѧ�Ծ��������棩 ���ͣ�ѡ����
1.28gͭͶ��һ������Ũ�����У�ͭ��ȫ�ܽ⣬����������ɫԽ��Խdz�����ռ���VmL���壨��״��������ʢ�д����������������ˮ�У�ͨ������ǡ��ʹ������ȫ�ܽ���ˮ�У�����Ҫ��״���µ��������Ϊ(����)
A. 504mL B. 336mL C. 224mL D. 168mL
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ʡ���IJ���ѧ2017�������ѧ��������ѧ�Ծ� ���ͣ������
19-I�����йؾ���ṹ�����ʵ���������ȷ���ǣ�______��
A.���д��ڼ��Լ������Ӽ������������
B.�������K>Na���ʽ����ص��۵���ڽ�����
C.��1mol�Ľ��ʯ��ʯī������������C-C������Ŀ��ͬ
D.����þ�ľ����ܴ����Ȼ��ƣ������۵�����Ȼ��ơ�
19-��ij������Ͻ�Ҳ��Ϊ������������磺Cu9Al4��Cu5Zn8�ȡ����ʴ��������⣺

(1)��̬пԭ�ӵĵ����Ų�ʽΪ_______________________________����֪����п������Ũ���ռ���Һ���ɿ����Ե����ǻ���п����Na2[Zn(OH)4]���������÷�Ӧ�����ӷ���ʽΪ�� ___________________________________________________����֪���ǻ���п�����ӿռ乹�������������ͣ���Zn2+���ӻ���ʽΪ__________________��
��2��ͭ����±��(SCN)2��Ӧ������Cu(SCN)2,1mol (SCN)2�����к���__________���Ҽ�����±��(SCN)2��Ӧ���������֣�A��������(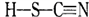 )��B-��������(
)��B-��������(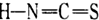 )������Ϊ��_________�������۵�ϸߵ���___________ (�����)��ԭ����________________________________��
)������Ϊ��_________�������۵�ϸߵ���___________ (�����)��ԭ����________________________________��
��3����֪��п������ͼ1��ʾ��������Zn2+����λ����____________�� S2-��ȡ�Ķѻ���ʽΪ____________________������A1��A2��A3��
��4����֪ͭ����γɵĽ���������Ľṹ��ͼ2��ʾ���������������ⳤΪa����(nm)���ý�����������ܶ�Ϊ_______g/cm3���ú�a��NA�Ĵ���ʽ��ʾ����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2016-2017ѧ���Ĵ�ʡ�ɶ��б���У���߶�3���¿���ѧ�Ծ��������棩 ���ͣ�ѡ����
��֪��H2(g)��1/2O2(g)===H2O(l) ��H1����285.8 kJ��mol��1����
H2(g)===H2(l)�� ��H2����0.92 kJ��mol��1 ��
O2(g)===O2(l)�� ��H3����6.84 kJ��mol��1 ��
H2O(g)===H2O(l)�� ��H4����44 kJ��mol��1 ��
����˵����ȷ���ǣ� ��
A. �����ĸ���Ӧ�������ȷ�Ӧ
B. 1 molҺ̬H2����������1 mol��̬H2������
C. H2��ȼ���Ȧ�HΪ��285.8 kJ��mol��1
D. �����Һ��ȼ�յ��Ȼ�ѧ����ʽΪH2(l)��1/2O2(l)===H2O(g)����H����285.8 kJ��mol��1
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com