
����Ŀ���״�����Ҫ�Ļ���ԭ�ϣ����úϳ�����CO��H2��CO2���ڴ����������ºϳɼ״������ܷ����ķ�Ӧ���£�
��CO2(g)��3H2(g)![]() CH3OH(g)��H2O(g) ��H1��-49.58kJ/mol K1
CH3OH(g)��H2O(g) ��H1��-49.58kJ/mol K1
��CO(g)��2H2(g)![]() CH3OH(g) ��H2��-90.77 kJ/mol K2
CH3OH(g) ��H2��-90.77 kJ/mol K2
��CO2(g)��H2(g)![]() CO(g)��H2O(g) ��H3 K3
CO(g)��H2O(g) ��H3 K3
��1����Ӧ�۵���H3��________����ѧƽ�ⳣ��K3��K1��K2�Ĵ�����ϵ��K3��_____��
��2��Ҫʹ��Ӧ�ڵ����ʺ�ת���ʶ�������Ҫ�ı��������___________����5MPa�£�Ҫ��߷�Ӧ�ڵ�ת���ʣ��ɲ�ȡ�Ĵ�ʩ��__________��_________������������
��3������Ӧ���ں����ܱ������н��У����п����жϸ÷�Ӧ�ﵽƽ�����_______�����ţ���
A��v��(H2)��v��(CH3OH) B�������ѹǿ����
C��c(H2)��c(H2O)��ֵ���� D��������ܶȲ���
��4����һ���¶Ⱥʹ��������£���1L�ܱ������г���1molCO2��3molH2������Ӧ�١���CO2��ƽ��ת����Ϊ50%ʱ������״����������Ϊ________�����¶��£�����Ӧ��ƽ�ⳣ��K��__________�������������ٳ���0.5molH2��0.5molH2O(g)��������������ʱƽ��_______�ƶ�������������������������������
���𰸡�+41.19 kJ/mol K1/K2 ��ѹ ���� ��ʱ������״� BC 16.7% 0.148 ����
��������
�Ÿ��ݸ�˹���ɵ�һ����Ӧ��ȥ�ڶ�����Ӧ�õ���Ӧ�ۣ�����ʽ�����ƽ�ⳣ�������
�Ʒ�Ӧ���������С�ķ��ȷ�Ӧ��һ��ѹǿ�£���Ӱ��ƽ���ƶ���������߷�Ӧ�ڵ�ת���ʵ�������
��A��v��(H2)��v��(CH3OH)��һ��һ�棬�����ʱȲ����ڼ���ϵ���ȣ�����˵���ﵽƽ�⣻B����Ӧ�������С�ķ�Ӧ������Ӧ��ѹǿ��С���������ѹǿ���䣬��ﵽƽ�⣻C��c(H2)��c(H2O)��ֵ���䣬��ﵽƽ�⣻D���ܶȵ�����������������������������������䣬����������䣬������ܶ�ʼ�ղ��䣬��˲�����Ϊ�ж�ƽ���־��
�Ƚ�������ʽ���������״���������������¶��£���������Ӧ��ƽ�ⳣ���������������ٳ���0.5 mol H2��0.5 mol H2O(g)������Ũ���̺�ƽ�ⳣ���Ƚϡ�
�Ÿ��ݸ�˹���ɵ�һ����Ӧ��ȥ�ڶ�����Ӧ�õ���Ӧ������H3��49.58 kJ��mol1(90.77 kJ��mol1) = +41.19 kJ��mol1������ʽ�����ƽ�ⳣ���������˻�ѧƽ�ⳣ��K3��K1��K2�Ĵ�����ϵ��K3��![]() ���ʴ�Ϊ��+41.19 kJ��mol1��
���ʴ�Ϊ��+41.19 kJ��mol1��![]() ��
��
�Ʒ�Ӧ���������С�ķ��ȷ�Ӧ��Ҫʹ��Ӧ�������ʺ�ת���ʶ�������Ҫ�ı�������Ǽ�ѹ����5 MPa�£�����ƽ�������ƶ�����ʱ�ķ�����״���ƽ�������ƶ���������߷�Ӧ����ת���ʣ��ʴ�Ϊ����ѹ�����¡���ʱ������״���
��A��v��(H2)��v��(CH3OH)��һ��һ�棬�����ʱȲ����ڼ���ϵ���ȣ�����˵���ﵽƽ�⣬��A���������⣻
B����Ӧ�������С�ķ�Ӧ������Ӧ��ѹǿ��С���������ѹǿ���䣬��ﵽƽ�⣬��B�������⣻
C��c(H2)��c(H2O)��ֵ���䣬��ﵽƽ�⣬��C�������⣻
D���ܶȵ�����������������������������������䣬����������䣬������ܶ�ʼ�ղ��䣬��˲�����Ϊ�ж�ƽ���־����D���������⡣
������������ΪBC��
����һ���¶Ⱥʹ��������£���1 L�ܱ������г���1 mol CO2��3 mol H2������Ӧ������CO2��ƽ��ת����Ϊ50%ʱ��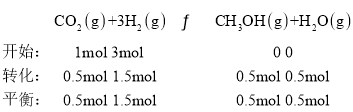 ������״����������Ϊ
������״����������Ϊ![]() �����¶��£�����Ӧ��ƽ�ⳣ��
�����¶��£�����Ӧ��ƽ�ⳣ��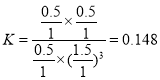 �������������ٳ���0.5 mol H2��0.5 mol H2O(g)��
�������������ٳ���0.5 mol H2��0.5 mol H2O(g)��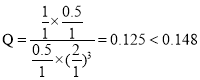 ��������������ʱƽ�������ƶ����ʴ�Ϊ��16.7%��0.148������
��������������ʱƽ�������ƶ����ʴ�Ϊ��16.7%��0.148������


 һ����ʦ�����Ծ�ϵ�д�
һ����ʦ�����Ծ�ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����֪ij��֬A����ϡ������������������ˮ�⣬����֬����Ͷ�Ԫ��B��B��������Ũ����������ͨ��������Ӧ�����л���D��
��1��д����֬A��ϡ������������������ˮ��Ļ�ѧ����ʽ��__��
��2����֪D��C��H��O��N����Ԫ����ɣ���Է�������Ϊ227��C��H��N�����������ֱ�Ϊ15.86%��2.20%��18.50%����D�ķ���ʽ��__��B��D�Ļ�ѧ����ʽ��__��
��3��C��B��������һ�������·�Ӧ���ɵĻ������Է�������Ϊ134��д��C���п��ܵĽṹ��ʽ��__��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��һ�ַ�����ά������ǿ�ȱȸ�˿�������㷺�����������ϡ���ṹƬ������ͼ
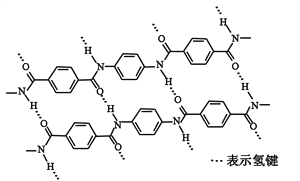
���й��ڸø߷��ӵ�˵����ȷ����
A. ��ȫˮ�����ĵ����������������ϵ���ԭ�Ӿ��в�ͬ�Ļ�ѧ����
B. ��ȫˮ�����ĵ��������������й������DCOOH���DNH2
C. ����Ըø߷��ӵ�����û��Ӱ��
D. �ṹ��ʽΪ��![]()
�鿴�𰸺ͽ���>>
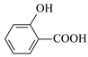
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���������ʵ����ʱȽϣ����۴������
A.Ӳ�ȣ����ʯ��̼���裾�����
B.���Ӱ뾶��S2-��Cl����Na+��O2-
C.�۵㣺NaF��NaCl��NaBr��NaI
D.�е㣺![]() ��
��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���¶�ΪT0ʱ��X(g)��Y(g)��2 L���ܱ������з�����Ӧ����Z(g)�������ʵ����ʵ�����ʱ��仯�Ĺ�ϵ��ͼa��ʾ������������ͬ���¶ȷֱ�ΪT1��T2ʱ������Ӧ��X�����ʵ�����ʱ��仯�Ĺ�ϵ��ͼb��ʾ�����������д������
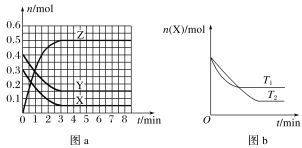
A.��Ӧ�ķ���ʽΪX(g)��Y(g)![]() 2Z(g)
2Z(g)
B.X(g)��Y(g)����Z(g)�ķ�Ӧ�Ƿ��ȷ�Ӧ
C.ͼa�з�Ӧ�ﵽƽ��ʱ��Y��ת����Ϊ62.5%
D.T1ʱ�����÷�Ӧ��ƽ�ⳣ��K��ֵΪ50����T1��T0
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ijѧϰС�����ⶨ���۳�֭������ά����C�ĺ�����ÿ100����ե��֭�к��д�Լ37.5���˵�ά����C��ʵ���ҿ��õ������ⶨ��֭������ά����C�ĺ�������Ӧ�ķ���ʽΪC6H8O6��I2==C6H6O6��2HI��ά����C��ѧʽΪC6H8O6����Է�������Ϊ176������ʵ�鲽�輰����������£�
�ٱ���Һ��ϡ�ͣ���ȡŨ��Ϊ0.0080mol/L�ĵ����Һ25.00mL��250mL����ƿ�У����ݣ�ҡ�ȱ��á�
����ȡ10.00mL������Ʒ�����ܶ�Ϊ1.0g/cm3����250 mL��ƿ�У�����50mL����ˮ��2mLָʾ����
���ڵζ�����װ��ϡ�ͺ�ı���Һ���ζ����յ㣬��ȡ����¼������ݡ�
���ظ��ⶨ3�Σ����ݼ�¼���±���
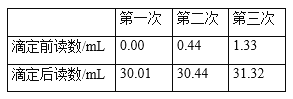
�ش��������⣺
��1��ʵ����ʢװ����ҺӦѡ��______��������ʽ��������ʽ�����ζ��ܡ�
��2������2�м����ָʾ����___________���жϵζ��ﵽ�յ��������__________��
��3��ʵ�������в������ܵ��²ⶨ���ƫ�͵���_______�����ţ���
A.ϡ�ͱ���Һ����ʱ���ӿ̶���
B.�ζ�����ʱ���Ӷ�
C.����ƿ�м�����Ʒ����ýϳ�ʱ��ſ�ʼ�ζ�
D.�ζ��ܼ��첿�������ݣ��ζ�����ʧ
��4�������������Ʒ��ά����C����Ϊ________mg/100 g���ú���______��������������������������ե��֭��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ������ϩ��һ��ʳ�����ϣ���ṹ��ʽ��ͼ��ʾ�������й�����ϩ�ķ�����ȷ���ǣ� ��
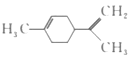
A.����һ�ȴ�����6��
B.���ķ��������е�̼ԭ��һ����ͬһƽ����
C.���Ͷ���������ͼ��ʾ����Ϊͬ���칹��![]()
D.һ�������£����ֱ���Է����ӳɡ�ȡ���������ȷ�Ӧ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ������Ȼ������гȻ���õ�廨��������ṹ��ʽ��ͼ�����ڸ��л������������ȷ����
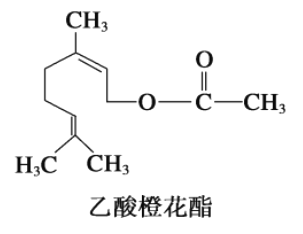
�� ��Ni��������1mol���л������3mol H2�����ӳ���
�� ���л��ﲻ�ܷ���������Ӧ��
�� ���л������ʽΪC12H22O2��
�� ���л����ͬ���칹���в������з��ࣻ
�� 1 mol���л���ˮ��ʱֻ������1 mol NaOH
A. �ڢۢ� B. �٢ܢ� C. �ڢܢ� D. �٢ڢ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�� (CH3)2CHCH2OH��ij����X�Ļ�ԭ�����X�������ǣ� ��
A.��ȩ��ͬϵ��B.��ȩ��ͬ���칹��C.CH2=C(CH3)CH2OHD.�Ҵ���ͬϵ��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com