
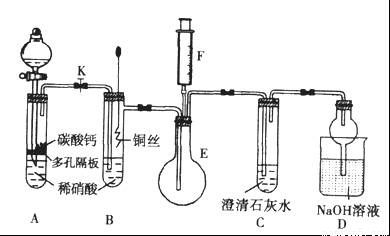
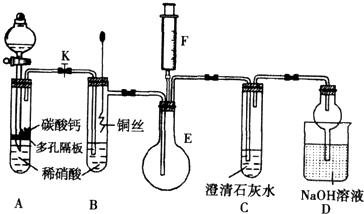
ijУ����С��Ϊ̽��ͭ��ϡ���ᷴӦ������������Ҫ��NO�����������ʵ�顣ͼ��KΪֹˮ�У�F��װ��һ�������ע���������м���װ�ú̶�װ�þ�����ȥ��
��ش��й����⣺
��1�����װ��A��Ŀ����_______________________��
��2�������װ��A��ʵ��Ŀ�ĺر�K���ٽ�װ��B�е�ͭ˿����ϡ���ᡣB�з�Ӧ�����ӷ���ʽ��____________________________��
��3����F�еĿ�������E�У�֤��NO���ڵ�ʵ��������_______________���˹��̷�����Ӧ�Ļ�ѧ����ʽ��____________________��
��4��װ��D��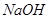 ��Һ��������________________��
��Һ��������________________��
��1���������ɵĶ�����̼�Ͼ�����װ���ڵĿ����������NO�ļ�����ɸ��š���2�֣�
��2��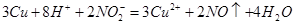 ��2�֣�
��2�֣�
��3��E����ɫ�����Ϊ����ɫ
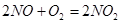 ��2�֣�
��2�֣�
��4�����ն���ĵ��������ֹ��Ⱦ������2�֣�
��������
���������A ��ȡ������̼���߿�������ֹ����һ�������ļ��顣B ����ȡһ��������װ�á�E һ������ת������������װ�á�C ��������̼��װ�á�D �����յ����������װ�á�
���㣺������һ����������ȡ��ת����ʵ�飬�����˵�������������ʡ�



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2014�콭��ʡ�����и�һ��ѧ�����п��Ի�ѧ�Ծ��������棩 ���ͣ�ʵ����
��11�֣� ijУ����С��Ϊ��̽��ͭ��ϡ���ᷴӦ������������Ҫ��NO�����������ʵ�飬װ����ͼ��ʾ(����װ�ú̶�װ�þ�����ȥ)��ͼ��KΪֹˮ��(���ڹر�״̬)��F��һ��յ�ע������

��ش��й����⣺
(1) ���װ��A��Ŀ���� ��
Ϊ�ﵽ��Ŀ�ģ�Ӧ���еIJ����Ǵ�K���Ҵ�Һ©����������װ��C�в���
ʱ���ر�K��
(2) �����(1)�еġ���������װ��B��ͭ˿����ϡ���ᣬ����֮���۲쵽װ��B�е�
������ ��
B�з�Ӧ�����ӷ���ʽΪ�� ��
(3) װ��E��F�������� ��Ϊʵ�ִ����ã������������ ��
(4) װ��D�����������ն���ĵ��������ֹ��Ⱦ���������� �Ĺ��ܡ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2014�찲��ʡ��һ��ѧ���������ʲ��Ի�ѧ�Ծ��������棩 ���ͣ�ʵ����
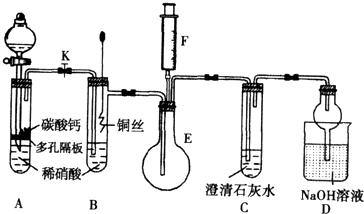
(12��) ijУ����С��Ϊ��̽��ͭ��ϡ���ᷴӦ������������Ҫ��NO�����������ʵ�飬װ����ͼ��ʾ(����װ�ú̶�װ�þ�����ȥ)��ͼ��KΪֹˮ��(���ڹر�״̬)��F��һ��յ�ע������

��ش��й����⣺
(1)���װ��A��Ŀ���� ��Ϊ�ﵽ��Ŀ�ģ�Ӧ���еIJ��� ��
(2)�����(1)�еġ���������װ��B��ͭ˿����ϡ���ᣬ����֮���۲쵽װ��B�е������� ��B�з�Ӧ�����ӷ���ʽΪ�� ��
(3)װ��E��F�������� ��Ϊʵ�ִ����ã������������ ��
(4)װ��D�������� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com