
��֪��2H2(g)��O2(g)==2H2O(l) ��H����571.6 kJ��mol��1
2CH3OH(l)��3O2(g)==2CO2(g)��4H2O(l) ��H����1452 kJ��mol��1
H��(aq)��OH��(aq)==H2O(l) ��H����57.3 kJ��mol��1
����˵����ȷ���ǣ� ��
A�� H2(g)��ȼ����Ϊ571.6 kJ��mol��1
H2(g)��ȼ����Ϊ571.6 kJ��mol��1
B��ͬ������H2(g)��CH3OH(l)��ȫȼ�գ�H2(g)�ų���������
C��1/2H2SO4(aq)��1/2Ba(OH)2(aq)== 1/2BaSO4(s)��H2O(l) ��H����57.3 kJ��mol��1
D��3H2(g)��CO2(g)=CH3OH(l)��H2O(l) ��H����135.9 kJ��mol��1

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ��2017�����ʡ������12���¿���ѧ�Ծ��������棩 ���ͣ������
�Իش��������⣺
(1)д��������������H2O2�����Ȼ����������ӷ�Ӧ����ʽ��_____________��
(2)�ճ������г��õġ�84����Һ������Ҫ�ɷ���NaClO������������Ҫ�ɷ���HCl�����߲��ܻ��ʹ�ã��������ӷ���ʽ��ʾԭ��_______________________��
(3)ȡ300 mL 0.2 mol/L KI��Һ��һ����������KMnO4��Һǡ����ȫ��Ӧ�����ɵ���������I2��KIO3��������KMnO4�����ʵ���Ϊ________mol
(4) ��Na2S2O3����Һ�м���ϡ����ʯ���������ǣ�______________��д���÷�Ӧ�����ӷ���ʽ_________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2017�����ʡ������12���¿���ѧ�Ծ��������棩 ���ͣ�ѡ����
��NAΪ�����ӵ�������ֵ��������NA�йص�˵����ȷ����
A���ڷ�ӦKClO3��6HCl��KCl��3Cl2����3H2O�У����õ���״����67.2 L Cl2ʱ����Ӧ��ת�Ƶĵ�����Ϊ6 NA
B��6.0 g SiO2�����к��еĹ�������ĿΪ0.2 NA
C����״���£�22.4 L NO��11.2 L O2��Ϻ�����ķ�������ΪNA
D��S2��S8�Ļ���ﹲ6.4 g������������ԭ����һ��Ϊ0.2 NA
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2016-2017ѧ��ɽ��ʡ�߶�12���¿���ѧ�Ծ��������棩 ���ͣ�ѡ����
����Һ����ʾ���Ĺ�����ʹ�õĻ�ѧ��ϴ��NF3��һ���������壬�䴢��������������CO2�ĵ�12000��20000�����ڴ����е������ɳ���740��֮�ã������Ǽ��ֻ�ѧ���ļ��ܣ�
��ѧ�� | N��N | F-F | N-F |
����/KJ��mol-1 | 941.7 | 154.8 | 283.0 |
����˵������ȷ����
A������N2(g)��2N(g)�ų�����
B������N(g)+3F(g)��NF3(g)�ų�����
C����ӦN2(g)+3F2(g)��2NF3(g)�ġ�H��0
D��NF3�������������û�л�ѧ���Ķ��������ɣ��Կ��ܷ�����ѧ��Ӧ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2016-2017ѧ��ɽ��ʡ�߶�12���¿���ѧ�Ծ��������棩 ���ͣ�ѡ����
�����£���10mL0.1mol/L������Һ�м�ˮϡ�ͺ�����˵����ȷ����
A����Һ�����ӵ���Ŀ��С
B���ټ���CH3COONa�����ܴٽ�����ĵ���
C������ĵ���̶�����c(H+)������
D����Һ��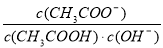 ����
����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2016-2017ѧ���ຣʡ�߶�11���¿���ѧ�Ծ��������棩 ���ͣ�ѡ����
����������ȷ����( )
A������Ӧ��Ũ����������Ч��ײ�ļ���
B������ѹǿ������Ӧ�����ӵİٷ���
C�����ӷ�Ӧ��Ũ��������Ӧ�����ӵİٷ���
D���ֽⷴӦ�������ȷ�Ӧ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2016-2017ѧ���ຣʡ�߶�11���¿���ѧ�Ծ��������棩 ���ͣ�ѡ����
2SO2(g)��O2(g) 2SO3(g)���Ʊ��������Ҫ��Ӧ������������ȷ����( )
2SO3(g)���Ʊ��������Ҫ��Ӧ������������ȷ����( )
A������V2O5���ı�÷�Ӧ���淴Ӧ����
B������Ӧ��ϵ��ѹǿ����Ӧ����һ������
C���÷�Ӧ�Ƿ��ȷ�Ӧ�������¶Ƚ����̷�Ӧ�ﵽƽ���ʱ��
D����t1��t2ʱ�̣�SO3(g)��Ũ�ȷֱ���c1��c2����ʱ����t1��t2�ڣ�SO3(g)���ɵ�ƽ������Ϊv��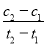
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2016-2017ѧ�����ɹŸ�һ���л�ѧ���������棩 ���ͣ�ѡ����
��H2�� N2��O2��������ֱ���벻ͬ�� ����ʹ�����¶ȡ��ܶ���ͬ������ѹǿ��p����С�Ĺ�ϵ����
����ʹ�����¶ȡ��ܶ���ͬ������ѹǿ��p����С�Ĺ�ϵ����
A��p��H2����p��O2����p��N2�� B��p��O2����P��N2����p��H2��
C��p��H2����P��N2����p��O2�� D��P��N2����p��O2����p��H2��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2016-2017ѧ��ӱ�ʡ�߶�11���¿���ѧ�Ծ��������棩 ���ͣ�ѡ����
���ݻ���ͬ�����ܱ�����A��B�У������¶�Ϊ423K��ͬʱ��A��B�зֱ����amol��b mol�⻯��(a>b)������Ӧ2HI(g) I2(g) + H2(g)��ƽ�������˵���϶���ȷ���ǣ���
I2(g) + H2(g)��ƽ�������˵���϶���ȷ���ǣ���
A�� �ӷ�Ӧ��ʼ������ƽ���ƽ�����ʣ�vA<vB
B�� ƽ��ʱI2��Ũ�ȣ�c(I2)A=c(I2)B
C�� ƽ��ʱ�������ڻ�������еİٷֺ��ã�A��������B����
D�� ƽ��ʱHI�ķֽ��ʣ���A=��B
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com