
Θ®14Ζ÷Θ© FeSO4?7H2OΙψΖΚ”Ο”Ύ“Ϋ“©ΚΆΙΛ“ΒΝλ”ρΓΘ
“‘œ¬ «FeSO4?7H2OΒΡ Β―ι “÷Τ±ΗΝς≥ΧΆΦΓΘΗυΨίΧβ“βΆξ≥…œ¬Ν–ΧνΩ’ΘΚ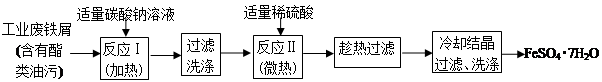
Θ®1Θ©ΧΦΥαΡΤ»ή“ΚΡή≥ΐ»ΞθΞάύ”ΆΈέΘ§ «“ρΈΣ Θ®”ΟάκΉ”ΖΫ≥Χ Ϋ±μ ΨΘ©Θ§Ζ¥”ΠΔώ–η“ΣΦ”»» ΐΖ÷÷”Θ§Τδ‘≠“ρ « ΓΘ
Θ®2Θ©ΖœΧζ–Φ÷–Κ§―θΜ·ΧζΘ§Έό–η‘Ύ÷Τ±Η«Α≥ΐ»ΞΘ§άμ”… «Θ®”ΟάκΉ”ΖΫ≥Χ ΫΜΊ¥πΘ© Θ§ ΓΘ
Θ®3Θ©≈–ΕœΖ¥”ΠΔρΆξ≥…ΒΡœ÷œσ «ΘΚ Θ§ ΓΘ
“‘œ¬ «≤βΕ®Ρ≥≤Ι―ΣΦΝΘ®FeSO4?7H2OΘ©÷–Χζ‘ΣΥΊΚ§ΝΩΒΡΝς≥ΧΆΦΓΘΗυΨίΧβ“βΆξ≥…œ¬Ν–ΧνΩ’ΘΚ
|
 Θ®4Θ©ΓΔ≤Ϋ÷ηΔσ–η“Σ100mL1mol/LΒΡœΓΝρΥαΘ§”Ο98.3%Θ§Π―=1.84g/cm3ΒΡ≈®ΝρΥα≈δ÷ΤΘ§Υυ”ΟΒΡ“«Τς”–ΝΩΆ≤ΓΔ…’±≠ΓΔ≤ΘΝßΑτΓΔΫΚΆΖΒΈΙήΦΑ ΓΘ–¥≥ω≤Ϋ÷ηΔτΒΡάκΉ”ΖΫ≥Χ ΫΘΚ ΓΘΘ®5Θ©≤Ϋ÷ηΔθ“ΜœΒΝ–≤ΌΉς“ά¥Έ «ΘΚΔΌΙΐ¬ΥΔΎœ¥Β”Δέ Δήά以Δί≥ΤΝΩΔόΚψ÷Ί≤ΌΉςΓΘ≤ΌΉςΔόΒΡΡΩΒΡ « ΓΘ
Θ®4Θ©ΓΔ≤Ϋ÷ηΔσ–η“Σ100mL1mol/LΒΡœΓΝρΥαΘ§”Ο98.3%Θ§Π―=1.84g/cm3ΒΡ≈®ΝρΥα≈δ÷ΤΘ§Υυ”ΟΒΡ“«Τς”–ΝΩΆ≤ΓΔ…’±≠ΓΔ≤ΘΝßΑτΓΔΫΚΆΖΒΈΙήΦΑ ΓΘ–¥≥ω≤Ϋ÷ηΔτΒΡάκΉ”ΖΫ≥Χ ΫΘΚ ΓΘΘ®5Θ©≤Ϋ÷ηΔθ“ΜœΒΝ–≤ΌΉς“ά¥Έ «ΘΚΔΌΙΐ¬ΥΔΎœ¥Β”Δέ Δήά以Δί≥ΤΝΩΔόΚψ÷Ί≤ΌΉςΓΘ≤ΌΉςΔόΒΡΡΩΒΡ « ΓΘΘ®1Θ©CO32ΓΣ+H2O HCO3ΓΣ+OHΓΣΘΜ…ΐΈ¬Θ§¥ΌΫχΥ°ΫβΘ§»ή“ΚΦν–‘‘ω«ΩΘ§ ΙΖ¥”Π≥δΖ÷Ϋχ––ΓΘ
HCO3ΓΣ+OHΓΣΘΜ…ΐΈ¬Θ§¥ΌΫχΥ°ΫβΘ§»ή“ΚΦν–‘‘ω«ΩΘ§ ΙΖ¥”Π≥δΖ÷Ϋχ––ΓΘ
Θ®2Θ©Fe2O3 +6H+Γζ2Fe3++3H2OΘ§ 2Fe3+ +Fe=3Fe2+
Θ®3Θ©ΧζΖέ≤Μ‘Ό»ήΫβΘ§ΧζΖέ±μΟφ≤Μ‘Ό”–Τχ≈ί≤ζ…ζ
Θ®4Θ©100mL»ίΝΩΤΩ Fe3++3OHΓΣ=Fe(OH)3Γΐ(ΚœάμΦ¥Ω…)
Θ®5Θ©ΔέΉΤ…’Θ®Φ”»»Θ© ΔόΚψ÷Ί≤ΌΉς»Ζ±Θ«β―θΜ·ΧζΆξ»ΪΖ÷Ϋβ≥…ΝΥ―θΜ·Χζ
Θ®6Θ©0.07a
ΫβΈω


 ΜΞΕ·ΩΈΧΟœΒΝ–¥πΑΗ
ΜΞΕ·ΩΈΧΟœΒΝ–¥πΑΗ ΦΛΜνΥΦΈ§÷«Ρή―ΒΝΖΩΈ ±ΒΦ―ßΝΖœΒΝ–¥πΑΗ
ΦΛΜνΥΦΈ§÷«Ρή―ΒΝΖΩΈ ±ΒΦ―ßΝΖœΒΝ–¥πΑΗ
| ΡξΦΕ | ΗΏ÷–ΩΈ≥Χ | ΡξΦΕ | ≥θ÷–ΩΈ≥Χ |
| ΗΏ“Μ | ΗΏ“ΜΟβΖ―ΩΈ≥ΧΆΤΦωΘΓ | ≥θ“Μ | ≥θ“ΜΟβΖ―ΩΈ≥ΧΆΤΦωΘΓ |
| ΗΏΕΰ | ΗΏΕΰΟβΖ―ΩΈ≥ΧΆΤΦωΘΓ | ≥θΕΰ | ≥θΕΰΟβΖ―ΩΈ≥ΧΆΤΦωΘΓ |
| ΗΏ»ΐ | ΗΏ»ΐΟβΖ―ΩΈ≥ΧΆΤΦωΘΓ | ≥θ»ΐ | ≥θ»ΐΟβΖ―ΩΈ≥ΧΆΤΦωΘΓ |
ΩΤΡΩΘΚΗΏ÷–Μ·―ß ά¥‘¥ΘΚ Χβ–ΆΘΚ‘ΡΕΝάμΫβ
≤ιΩ¥¥πΑΗΚΆΫβΈω>>
ΩΤΡΩΘΚΗΏ÷–Μ·―ß ά¥‘¥ΘΚ Χβ–ΆΘΚ
ΔΌΫΪ5%Na2CO3»ή“ΚΦ”»κΒΫ Δ”–“ΜΕ®ΝΩΖœΧζ–ΦΒΡ…’±≠÷–Θ§Φ”»» ΐΖ÷÷”Θ§”Ο«ψΈωΖ®≥ΐ»ΞNa2CO3»ή“ΚΘ§»ΜΚσΫΪΖœΧζ–Φ”ΟΥ°œ¥Β”2ΓΣ3±ιΘΜ
ΔΎœρœ¥Β”ΙΐΒΡΖœΧζ–Φ÷–Φ”»κΙΐΝΩΒΡœΓΝρΥαΘ§ΩΊ÷ΤΈ¬Ε»‘Ύ50 ΓφΓΣ80 Γφ÷°Φδ÷ΝΧζ–ΦΚΡΨΓΘΜ
Δέ≥Ο»»Ιΐ¬ΥΘ§ΫΪ¬Υ“ΚΉΣ»κΒΫΟή±’»ίΤς÷–Θ§Ψ≤÷ΟΓΔά以ΫαΨßΘΜ
Δή¥ΐΫαΨßΆξ±œΚσΘ§¬Υ≥ωΨßΧεΘ§”Ο…ΌΝΩ±υΥ°œ¥Β”2ΓΣ3¥ΈΘ§‘Ό”Ο¬Υ÷ΫΫΪΨßΧεΈϋΗ…ΘΜ
ΔίΫΪ÷ΤΒΟΒΡFeSO4ΓΛ7H2OΨßΧεΖ≈‘Ύ“ΜΗω–ΓΙψΩΎΤΩ÷–Θ§Οή±’±Θ¥φΓΘ
«κΆξ≥…œ¬Ν–Έ ΧβΘΚ
Θ®1Θ© Β―ι≤Ϋ÷ηΔΌΒΡΡΩΒΡ «_________________Θ§Φ”»»ΒΡΉς”Ο «__________________________ΓΘ
Θ®2Θ© Β―ι≤Ϋ÷ηΔΎΟςœ‘≤ΜΚœάμΘ§άμ”… «______________________________________________ΓΘ
Θ®3Θ© Β―ι≤Ϋ÷ηΔή÷–”Ο…ΌΝΩ±υΥ°œ¥Β”ΨßΧεΘ§ΤδΡΩΒΡ «_________________ΘΜ_________________ΓΘ
Θ®4Θ©Ψ≠≤ι‘ΡΉ ΝœΚσΖΔœ÷Θ§ΝρΥα―«Χζ‘Ύ≤ΜΆ§Έ¬Ε»œ¬ΫαΨßΩ…Ζ÷±πΒΟΒΫFeSO4ΓΛ7H2OΓΔFeSO4ΓΛ4H2OΚΆFeSO4ΓΛH2OΓΘΝρΥα―«Χζ‘Ύ≤ΜΆ§Έ¬Ε»œ¬ΒΡ»ήΫβΕ»ΚΆΗΟΈ¬Ε»œ¬Έω≥ωΨßΧεΒΡΉι≥…»γœ¬±μΥυ ΨΘ®Ϋω‘Ύ56.7 ΓφΓΔ64 ΓφΈ¬Ε»œ¬Ω…Ά§ ±Έω≥ωΝΫ÷÷ΨßΧεΘ©ΓΘ
ΝρΥα―«ΧζΒΡ»ήΫβΕ»ΚΆΈω≥ωΨßΧεΒΡΉι≥…
Έ¬Ε»/Γφ | 0 | 10 | 30 | 50 | 56.7 | 60 | 64 | 70 | 80 | 90 |
»ήΫβΕ»/g | 14.0 | 17.0 | 25.0 | 33.0 | 35.2 | 35.3 | 35.6 | 33.0 | 30.5 | 27.0 |
Έω≥ωΨßΧε | FeSO4ΓΛ7H2O | FeSO4ΓΛ4H2O | FeSO4ΓΛH2O | |||||||
«κΗυΨί±μ÷– ΐΨίΉς≥ωΝρΥα―«ΧζΒΡ»ήΫβΕ»«ζœΏΓΘ
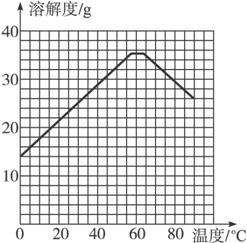
Θ®5Θ©»τ–η¥”ΝρΥα―«Χζ»ή“Κ÷–ΫαΨß≥ωFeSO4ΓΛ4H2OΘ§”ΠΩΊ÷ΤΒΡΫαΨßΈ¬Ε»Θ®tΘ©ΒΡΖΕΈßΈΣ_________________ΓΘ
≤ιΩ¥¥πΑΗΚΆΫβΈω>>
ΩΤΡΩΘΚΗΏ÷–Μ·―ß ά¥‘¥ΘΚ Χβ–ΆΘΚ‘ΡΕΝάμΫβ
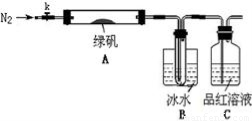
ΝρΥα―«ΧζΘ®FeSO4ΓΛ7H2O) «“Μ÷÷÷Ί“ΣΒΡ ≥ΤΖΚΆΥ«ΝœΧμΦ”ΦΝΓΘ Β―ι “Ά®Ιΐ»γœ¬ Β―ι”…ΖœΧζ–Φ÷Τ±ΗFeSO4ΓΛ7H2OΨßΧεΘΚ
ΔΌΫΪ5% Na2CO3»ή“ΚΦ”»κΒΫ Δ”–“ΜΕ®ΝΩΖœΧζ–ΦΒΡ…’±≠÷–Θ§Φ”»» ΐΖ÷÷”Θ§”Ο«ψΈωΖ®≥ΐ»ΞNa2CO3»ή“ΚΘ§»ΜΚσΫΪΖœΧζ–Φ”ΟΥ°œ¥Β”2ΓΪ3±ιΘΜΔΎœρœ¥Β”ΙΐΒΡΖœΧζ–Φ÷–Φ”»κΙΐΝΩΒΡœΓΝρΥαΘ§ΩΊ÷ΤΈ¬Ε»‘Ύ50ΓΪ80 Γφ÷°Φδ÷ΝΧζ–ΦΚΡΨΓΘΜΔέ≥Ο»»Ιΐ¬ΥΘ§ΫΪ¬Υ“ΚΉΣ»κΒΫΟή±’»ίΤς÷–Θ§Ψ≤÷ΟΓΔά以ΫαΨßΘΜΔή¥ΐΫαΨßΆξ±œΚσΘ§¬Υ≥ωΨßΧεΘ§”Ο…ΌΝΩ±υΥ°œ¥Β”2ΓΪ3¥ΈΘ§‘Ό”Ο¬Υ÷ΫΫΪΨßΧεΈϋΗ…ΘΜΔίΫΪ÷ΤΒΟΒΡFeSO4ΓΛ7H2OΨßΧεΖ≈‘Ύ“ΜΗω–ΓΙψΩΎΤΩ÷–Θ§Οή±’±Θ¥φΓΘ
«κΆξ≥…œ¬Ν–Έ ΧβΘΚ
Θ®1Θ© Β―ι≤Ϋ÷ηΔΌΒΡΡΩΒΡ «___________________Θ§Φ”»»ΒΡΉς”Ο «
_________________________ΓΘ
Θ®2Θ© Β―ι≤Ϋ÷ηΔΎΟςœ‘≤ΜΚœάμΘ§άμ”… «
________________________________________________ΓΘ
Θ®3Θ© Β―ι≤Ϋ÷ηΔή÷–”Ο…ΌΝΩ±υΥ°œ¥Β”ΨßΧεΘ§ΤδΡΩΒΡ «_________________ΘΜ
__________________ΓΘ
Θ®4Θ©Ψ≠≤ι‘ΡΉ ΝœΚσΖΔœ÷Θ§ΝρΥα―«Χζ‘Ύ≤ΜΆ§Έ¬Ε»œ¬ΫαΨßΩ…Ζ÷±πΒΟΒΫFeSO4ΓΛ7H2OΓΔFeSO4ΓΛ4H2OΚΆFeSO4ΓΛH2OΓΘΝρΥα―«Χζ‘Ύ≤ΜΆ§Έ¬Ε»œ¬ΒΡ»ήΫβΕ»ΚΆΗΟΈ¬Ε»œ¬Έω≥ωΨßΧεΒΡΉι≥…»γœ¬±μΥυ ΨΘ®Ϋω‘Ύ56.7 ΓφΓΔ64 ΓφΈ¬Ε»œ¬Ω…Ά§ ±Έω≥ωΝΫ÷÷ΨßΧεΘ©ΓΘ
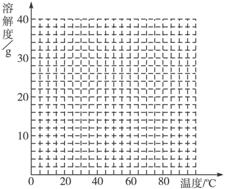
ΝρΥα―«ΧζΒΡ»ήΫβΕ»ΚΆΈω≥ωΨßΧεΒΡΉι≥…
| Έ¬Ε»/Γφ | 0 | 10 | 30 | 50 | 56.7 | 60 | 64 | 70 | 80 | 90 |
| »ήΫβΕ»/g | 14.0 | 17.0 | 25.0 | 33.0 | 35.2 | 35.3 | 35.6 | 33.0 | 30.5 | 27.0 |
| Έω≥ωΨßΧε | FeSO4ΓΛ7H2O | FeSO4ΓΛ4H2O | FeSO4ΓΛH2O | |||||||
«κΗυΨί±μ÷– ΐΨίΉς≥ωΝρΥα―«ΧζΒΡ»ήΫβΕ»«ζœΏΓΘ
Θ®5Θ©»τ–η¥”ΝρΥα―«Χζ»ή“Κ÷–ΫαΨß≥ωFeSO4ΓΛ4H2OΘ§”ΠΩΊ÷ΤΒΡΫαΨßΈ¬Ε»Θ®tΘ©ΒΡΖΕΈßΈΣ________ΓΘ
≤ιΩ¥¥πΑΗΚΆΫβΈω>>
ΩΤΡΩΘΚΗΏ÷–Μ·―ß ά¥‘¥ΘΚ Χβ–ΆΘΚ
(10Ζ÷) ΝρΥα―«ΧζΘ®FeSO4ΓΛ7H2OΘ© «“Μ÷÷÷Ί“ΣΒΡ ≥ΤΖΚΆΥ«ΝœΧμΦ”ΦΝΓΘ Β―ι “Ά®Ιΐ»γœ¬ Β―ι”…ΖœΧζ–Φ÷Τ±ΗFeSO4ΓΛ7H2OΨßΧεΘΚΔΌΫΪ5% Na2CO3»ή“ΚΦ”»κΒΫ Δ”–“ΜΕ®ΝΩΖœΧζ–ΦΒΡ…’±≠÷–Θ§Φ”»» ΐΖ÷÷”Θ§”Ο«ψΈωΖ®≥ΐ»ΞNa2CO3»ή“ΚΘ§»ΜΚσΫΪΖœΧζ–Φ”ΟΥ°œ¥Β”2 ΓΪ 3±ιΘΜΔΎœρœ¥Β”ΙΐΒΡΖœΧζ–Φ÷–Φ”»κΙΐΝΩΒΡœΓΝρΥαΘ§ΩΊ÷ΤΈ¬Ε»‘Ύ50 ΓΪ 80Γφ÷°Φδ÷ΝΧζ–ΦΚΡΨΓΘΜΔέ≥Ο»»Ιΐ¬ΥΘ§ΫΪ¬Υ“ΚΉΣ»κΒΫΟή±’»ίΤς÷–Θ§Ψ≤÷ΟΓΔά以ΫαΨßΘΜΔή¥ΐΫαΨßΆξ±œΚσΘ§¬Υ≥ωΨßΧεΘ§”Ο…ΌΝΩ±υΥ°œ¥Β”2 ΓΪ 3¥ΈΘ§‘Ό”Ο¬Υ÷ΫΫΪΨßΧεΈϋΗ…ΘΜΔίΫΪ÷ΤΒΟΒΡFeSO4ΓΛ7H2OΨßΧεΖ≈‘Ύ“ΜΗω–ΓΙψΩΎΤΩ÷–Θ§Οή±’±Θ¥φΓΘ«κΜΊ¥πœ¬Ν–Έ ΧβΘΚ
(1)–¥≥ωFe2ΘΪΒΡΚΥΆβΒγΉ”≈≈≤Φ Ϋ
(2)”ΟΙλΒά±μ Ψ Ϋ±μ ΨFe3ΘΪΒΡ3dΒγΉ”ΒΡ≈≈≤Φ«ιΩω
(3)ΝρΥα―«Χζ÷Τ±Η÷– Β―ι≤Ϋ÷ηΔΌΒΡΡΩΒΡ « ΓΓΓΓ ΓΘ
(4) Β―ι≤Ϋ÷ηΔΎΟςœ‘≤ΜΚœάμΘ§άμ”… « ΓΘ
(5) Β―ι≤Ϋ÷ηΔή÷–”Ο…ΌΝΩ±υΥ°œ¥Β”ΨßΧεΘ§ΤδΡΩΒΡ « ΓΘ
≤ιΩ¥¥πΑΗΚΆΫβΈω>>
ΩΤΡΩΘΚΗΏ÷–Μ·―ß ά¥‘¥ΘΚ2013-2014―ßΡξΫ≠Ές ΓΑΥ–ΘΗΏ»ΐœ¬―ßΤΎΝΣΩΦάμΉέΜ·―ß ‘ΨμΘ®ΫβΈωΑφΘ© Χβ–ΆΘΚ Β―ιΧβ
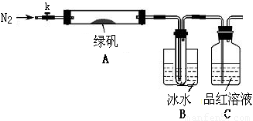
¬ΧΖ·Θ®FeSO4?7H2OΘ© «÷ΈΝΤ»±Χζ–‘ΤΕ―ΣΒΡΧΊ–ß“©ΓΘΡ≥―ß–ΘΒΡΜ·―ß–Υ»Λ–ΓΉιΒΡΆ§―ßΕ‘¬ΧΖ·Ϋχ––ΝΥ»γœ¬ΒΡΧΫΨΩΘΚ
FeSO4?7H2OΒΡ÷Τ±Η
ΗΟΜ·―ß–Υ»Λ–ΓΉιΒΡΆ§―ß‘Ύ Β―ι “Ά®Ιΐ»γœ¬ Β―ι”…ΖœΧζ–ΦΘ®Κ§…ΌΝΩ―θΜ·Ά≠ΓΔ―θΜ·ΧζΒ»‘”÷ Θ©÷Τ±ΗFeSO4ΓΛ7H2OΨßΧεΘΚ
ΔΌΫΪ5%Na2CO3»ή“ΚΦ”»κΒΫ Δ”–“ΜΕ®ΝΩΖœΧζ–ΦΒΡ…’±≠÷–Θ§Φ”»» ΐΖ÷÷”Θ§”Ο«ψΈωΖ®≥ΐ»Ξ
Na2CO3»ή“ΚΘ§»ΜΚσΫΪΖœΧζ–Φ”ΟΥ°œ¥Β”2ΓΪ3±ιΓΘ
ΔΎœρœ¥Β”ΙΐΒΡΖœΧζ–Φ÷–Φ”»κΙΐΝΩΒΡœΓΝρΥαΘ§ΩΊ÷ΤΈ¬Ε»‘Ύ50ΓΪ80Γφ÷°Φδ÷ΝΧζ–ΦΚΡΨΓΘΜ
Δέ≥Ο»»Ιΐ¬ΥΘ§ΫΪ¬Υ“ΚΉΣ»κΒΫΟή±’»ίΤς÷–Θ§Ψ≤÷ΟΓΔά以ΫαΨßΘΜ
Δή¥ΐΫαΨßΆξ±œΚσΘ§¬Υ≥ωΨßΧεΘ§”Ο…ΌΝΩ±υΥ°œ¥Β”2ΓΪ3¥ΈΘ§‘Ό”Ο¬Υ÷ΫΫΪΨßΧεΈϋΗ…ΘΜ
ΔίΫΪ÷ΤΒΟΒΡFeSO4ΓΛ7H2OΨßΧεΖ≈‘Ύ“ΜΗω–ΓΙψΩΎΤΩ÷–Θ§Οή±’±Θ¥φΓΘ
«κΜΊ¥πœ¬Ν–Έ ΧβΘΚ
Θ®1Θ© Β―ι≤Ϋ÷ηΔΌΒΡΡΩΒΡ «ΓΓΓΓΓΓΓΓΓΓΓΓΓΓΓΓΓΓΓΓΓΓΓΓΓΓΓΘ
Θ®2Θ© Β―ι≤Ϋ÷ηΔΎΟςœ‘≤ΜΚœάμΘ§άμ”… «ΓΓΓΓΓΓΓΓΓΓΓΓΓΓΓΓΓΓΓΓΓΓΓΓΓΘ
Θ®3Θ©ΈΣΝΥœ¥Β”≥ΐ»ΞΨßΧε±μΟφΗΫΉ≈ΒΡΝρΥαΒ»‘”÷ Θ§ Β―ι≤Ϋ÷ηΔή÷–”Ο…ΌΝΩ±υΥ°œ¥Β”ΨßΧεΘ§‘≠“ρ «ΓΓΓΓΓΓΓΓΓΓΓΓΓΓΓΓΓΓΓΓΓΓΓΓΓΓΓΓΓΓΓΓΓΓΓΓΓΓ ΓΘ
Θ®ΕΰΘ©ΧΫΨΩ¬ΧΖ·Θ®FeSO4ΓΛ7H2OΘ©»»Ζ÷ΫβΒΡ≤ζΈο
 “―÷ΣSO3ΒΡ»έΒψ «16.8ΓψCΘ§Ζ–Βψ «44.8ΓψCΘ§ΗΟ–ΓΉι…ηΦΤ»γœ¬ΆΦΥυ ΨΒΡ Β―ιΉΑ÷ΟΘ®ΆΦ÷–Φ”»»ΓΔΦ–≥÷“«ΤςΒ»Ψυ Γ¬‘Θ©ΘΚ
“―÷ΣSO3ΒΡ»έΒψ «16.8ΓψCΘ§Ζ–Βψ «44.8ΓψCΘ§ΗΟ–ΓΉι…ηΦΤ»γœ¬ΆΦΥυ ΨΒΡ Β―ιΉΑ÷ΟΘ®ΆΦ÷–Φ”»»ΓΔΦ–≥÷“«ΤςΒ»Ψυ Γ¬‘Θ©ΘΚ
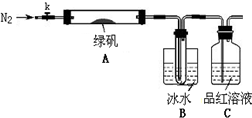
ΓΨ Β―ιΙΐ≥ΧΓΩ
ΔΌ“«ΤςΝ§Ϋ”ΚσΘ§Φλ≤ιΉΑ÷ΟA”κBΤχΟή–‘ΘΜ
ΔΎ»Γ“ΜΕ®ΝΩ¬ΧΖ·ΙΧΧε÷Ο”ΎA÷–Θ§Ά®»κN2“‘«ΐΨΓΉΑ÷ΟΡΎΒΡΩ’ΤχΘ§ΙΊ±’kΘ§”ΟΨΤΨΪΒΤΦ”»»”≤÷ ≤ΘΝßΙήΘΜ
ΔέΙέ≤λΒΫA ÷–ΙΧΧε÷πΫΞ±δΚλΉΊ…ΪΘ§B÷– ‘Ιή ’Φ·ΒΫΈό…Ϊ“ΚΧεΘ§C÷–»ή“ΚΆ …ΪΘΜ
Δή¥ΐA÷–Ζ¥”ΠΆξ»Ϊ≤Δά以÷Ν “Έ¬ΚσΘ§»Γ…ΌΝΩΖ¥”ΠΚσΙΧΧε”Ύ ‘Ιή÷–Θ§Φ”»κΝρΥα»ήΫβΘ§»Γ…ΌΝΩΒΈ»κΦΗΒΈKSCN»ή“ΚΘ§»ή“Κ±δΚλ…ΪΘΜ
ΔίΆυBΉΑ÷ΟΒΡ ‘Ιή÷–ΒΈ»κΦΗΒΈBaCl2»ή“ΚΘ§»ή“Κ±δΜκΉ«ΓΘ
(4Θ© Β―ιΫαΙϊΖ÷Έω
Ϋα¬έ1ΘΚB÷– ’Φ·ΒΫΒΡ“ΚΧε «?????????????????? ΘΜ
Ϋα¬έ2ΘΚC÷–»ή“ΚΆ …ΪΘ§Ω…ΆΤ÷Σ≤ζΈο÷–”–???? ?????????????? ΘΜ
Ϋα¬έ3ΘΚΉέΚœΖ÷Έω…œ ω Β―ιΔέΚΆΔήΩ…ΆΤ÷ΣΙΧΧε≤ζΈο“ΜΕ®”–Fe2O3ΓΘ
ΓΨ Β―ιΖ¥ΥΦΓΩ
Θ®5Θ©«κ÷Η≥ωΗΟ–ΓΉι…ηΦΤΒΡ Β―ιΉΑ÷ΟΒΡΟςœ‘≤ΜΉψΘΚ??????????????????????????? ΓΘ
Θ®6Θ©Ζ÷ΫβΚσΒΡΙΧΧε÷–Ω…ΡήΚ§”–…ΌΝΩFeOΘ§»Γ…œ ω Β―ιΔή÷–―ΈΥα»ήΫβΚσΒΡ»ή“Κ…Ό–μ”Ύ ‘Ιή÷–Θ§―ÔϓΜ÷÷ ‘ΦΝΦχ±πΘ§ΗΟ ‘ΦΝΉνΚœ ΒΡ «?????????? ΓΘ
aΘ°¬»Υ°ΚΆKSCN»ή“Κ???? bΘ°Υα–‘KMnO4»ή“Κ????? cΘ°H2O2???? dΘ°NaOH»ή“Κ
≤ιΩ¥¥πΑΗΚΆΫβΈω>>
ΙζΦ ―ß–Θ”≈―Γ - ΝΖœΑ≤αΝ–±μ - ‘ΧβΝ–±μ
Κΰ±± ΓΜΞΝΣΆχΈΞΖ®ΚΆ≤ΜΝΦ–≈œΔΨΌ±®ΤΫΧ® | Άχ…œ”–ΚΠ–≈œΔΨΌ±®Ή®«χ | Βγ–≈’©Τ≠ΨΌ±®Ή®«χ | …φάζ Ζ–ιΈό÷ς“ε”–ΚΠ–≈œΔΨΌ±®Ή®«χ | …φΤσ«÷»®ΨΌ±®Ή®«χ
ΈΞΖ®ΚΆ≤ΜΝΦ–≈œΔΨΌ±®ΒγΜΑΘΚ027-86699610 ΨΌ±®” œδΘΚ58377363@163.com