
(20��)ijͬѧ�÷ϸɵ���ڵĺ�ɫ����(���ܺ���MnO2��NH4Cl��ZnCl2������)������ͼ��ʾʵ�飺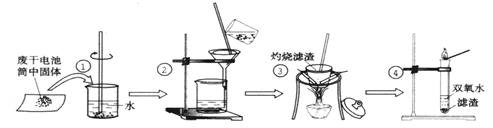
(1)��������������ʱ������Ҫ�����оƾ��ơ��������� �� ��
�ȣ�ֻ���������������ɣ���
(2)�����ܵ��Թܼ�����������������Թ���Ѹ�ٲ�����ʹ�����ǵ�ľ����ȼ�����壬�ɴ��Ʋ���������ΪMnO2���Թ��з�����Ӧ�Ļ�ѧ����ʽ�� ��
(3)���������������еĺ�ɫ����ʱ������һ��ʹ����ʯ��ˮ����ǵ����壬�ɴ��Ʋ������г�����MnO2�⣬�����ڵ�����Ϊ�� ��
(4)��֪�Ȼ�п��ϡ��ˮ��Ӧ������Zn(OH)2��ɫ������Zn(O H)2��������ϡ��ˮ���ɿ����Ե�Zn(NH3)4(OH)2�������Ǹ�ͬѧ�Բ����ڵ���Һ���γɷֽ���̽���Ĺ��̣�
H)2��������ϡ��ˮ���ɿ����Ե�Zn(NH3)4(OH)2�������Ǹ�ͬѧ�Բ����ڵ���Һ���γɷֽ���̽���Ĺ��̣�
[����I]����Ҫ�ɷ�Ϊ�Ȼ�泥�
[��֤������������]��ȡ������Һ����NaOH���壬�����ȣ�
[ʵ������]���ŵ������İ�ζ��
[�жϲ���]�� �������I����������I������������
[����II]����Ҫ�ɷ�Ϊ�Ȼ�п��
[��֤������������]�� ��
[ʵ������]�� ��
[�жϲ���]������������������ʵ��ó����ۣ�
�����ڵ���Һ�е���Ҫ�ɷ�Ϊ�� ����Ҫ�ɷ�Ϊ�� ��

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

A���
�Իش��������⣺
(1)��д�������������Ƶ�Ī���εĻ�ѧ����ʽ��_________________________________��
(2)�μ�ϡ����ʱ�������䷴Ӧ���ʱ�ͬŨ�������봿���۷�Ӧ�죬��ԭ����___________
_____________________________________��
(3)ȡ
����ƽ���з�Ӧʽ��
______Fe2++______![]() +______H+����______Mn2++______Fe3++______H2O
+______H+����______Mn2++______Fe3++______H2O
����________(��ʽ���ʽ)�ζ���ʢװ���������Һ��
�۸��������Һ�����ʵ���Ũ����________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
(20��)ijͬѧ�÷ϸɵ���ڵĺ�ɫ����(���ܺ���MnO2��NH4Cl��ZnCl2������)������ͼ��ʾʵ�飺
(1)��������������ʱ������Ҫ�����оƾ��ơ��������� �� ��
�ȣ�ֻ���������������ɣ���
(2)�����ܵ��Թܼ�����������������Թ���Ѹ�ٲ�����ʹ�����ǵ�ľ����ȼ�����壬�ɴ��Ʋ���������ΪMnO2���Թ��з�����Ӧ�Ļ�ѧ����ʽ�� ��
(3)���������������еĺ�ɫ����ʱ������һ��ʹ����ʯ��ˮ����ǵ����壬�ɴ��Ʋ������г�����MnO2�⣬�����ڵ�����Ϊ�� ��
(4)��֪�Ȼ�п��ϡ��ˮ��Ӧ������Zn(OH)2��ɫ������Zn(OH)2��������ϡ��ˮ���ɿ����Ե�Zn(NH3)4(OH)2�������Ǹ�ͬѧ�Բ����ڵ���Һ���γɷֽ���̽���Ĺ��̣�
[����I]����Ҫ�ɷ�Ϊ�Ȼ�泥�
[��֤������������]��ȡ������Һ����NaOH���壬�����ȣ�
[ʵ������]���ŵ������İ�ζ��
[�жϲ���]�� �������I����������I������������
[����II]����Ҫ�ɷ�Ϊ�Ȼ�п��
[��֤������������]�� ��
[ʵ������]�� ��
[�жϲ���]������������������ʵ��ó����ۣ�
�����ڵ���Һ�е���Ҫ�ɷ�Ϊ�� ����Ҫ�ɷ�Ϊ�� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012-2013ѧ������ʡ��Ϫһ�и߶��ڶ����¿���ѧ�Ծ����������� ���ͣ�ʵ����
(14��)��������ͭ�Ƚ������仯�������ճ�������Ӧ�ù㷺�����������ʵ��ش����⣺
(1)�����к���һ����̼������X(Fe3C)��X�������Ŀ����и������գ������д��ԵĹ���Y����Y���ڹ����������Һ�к��еĴ�����������________________________��
(2)ij��Һ����Mg2����Fe2����Al3����Cu2�����������ӣ������м��������NaOH��Һ���ˣ��������������ղ������պ�Ĺ���Ͷ�뵽������ϡ�����У�������Һ��ԭ��Һ��ȣ���Һ�д������ٵ���������________��
A��Mg2������B��Fe2�� C��Al3�� D��Cu2��
(3)����������Ҫ��ҵ���ϣ��÷���м�Ʊ������������£�
�ش��������⣺
�ٲ������������________���������������________��
��д���ڿ���������FeCO3�Ļ�ѧ����ʽ ��
(4)��Щͬѧ��ΪKMnO4��Һ�ζ�Ҳ�ܽ�����Ԫ�غ����IJⶨ(5Fe2����MnO4����8H��===5Fe3����Mn2����4H2O)��
a.��ȡ2.850g�̷���FeSO4��7H2O����Ʒ���ܽ⣬��250mL����ƿ�ж��ݣ�
b.��ȡ25.00mL������Һ����ƿ�У�
c.�������ữ��0.01000mol/LKMnO4��Һ�ζ����յ㣬����KMnO4��Һ�����ƽ��ֵΪ20.00mL��
��ʵ��ǰ������Ҫ��ȷ����һ�����ʵ���Ũ�ȵ�KMnO4��Һ250 mL������ʱ��Ҫ����������ƽ�����������ձ�����ͷ�ι��⣬����_____________________________________________��
��ijͬѧ��Ƶ����еζ���ʽ�����������________��(�гֲ�����ȥ)(����ĸ���)
�ۼ���������Ʒ��FeSO4��7H2O����������Ϊ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2010-2011ѧ�������ʡ������ѧ����ĩ���Ի�ѧ�Ծ� ���ͣ�ʵ����
(20��)ijͬѧ�÷ϸɵ���ڵĺ�ɫ����(���ܺ���MnO2��NH4Cl��ZnCl2������)������ͼ��ʾʵ�飺

(1)��������������ʱ������Ҫ�����оƾ��ơ��������� �� ��
�ȣ�ֻ���������������ɣ���
(2)�����ܵ��Թܼ�����������������Թ���Ѹ�ٲ�����ʹ�����ǵ�ľ����ȼ�����壬�ɴ��Ʋ���������ΪMnO2���Թ��з�����Ӧ�Ļ�ѧ����ʽ�� ��
(3)���������������еĺ�ɫ����ʱ������һ��ʹ����ʯ��ˮ����ǵ����壬�ɴ��Ʋ������г�����MnO2�⣬�����ڵ�����Ϊ�� ��
(4)��֪�Ȼ�п��ϡ��ˮ��Ӧ������Zn(OH)2��ɫ������Zn(OH)2��������ϡ��ˮ���ɿ����Ե�Zn(NH3)4(OH)2�������Ǹ�ͬѧ�Բ����ڵ���Һ���γɷֽ���̽���Ĺ��̣�
[����I]����Ҫ�ɷ�Ϊ�Ȼ�泥�
[��֤������������]��ȡ������Һ����NaOH���壬�����ȣ�
[ʵ������]���ŵ������İ�ζ��
[�жϲ���]�� �������I����������I������������
[����II]����Ҫ�ɷ�Ϊ�Ȼ�п��
[��֤������������]�� ��
[ʵ������]�� ��
[�жϲ���]������������������ʵ��ó����ۣ�
�����ڵ���Һ�е���Ҫ�ɷ�Ϊ�� ����Ҫ�ɷ�Ϊ�� ��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com