
����Ŀ������̼��ˮ�����Ʊ�ˮú���ĺ��ķ�ӦΪ��C(s)��H2O(g)H2(g)��CO(g)
(1)��֪̼(ʯī)��H2��CO��ȼ���ȷֱ�Ϊ393.5kJ��mol��1��285.8kJ��mol��1��283kJ��mol��1����֪H2O(l)=H2O(g)����H����44kJ��mol��1����C(s)��H2O(g)CO(g)��H2(g)����H��___��
(2)��ij�¶��£������Ϊ1L�ĺ����ܱո��������м�����������̿��������1mol H2O(g)����������Ӧ����Ӧʱ����������������ѹǿ�����������
ʱ��/min | 0 | 10 | 20 | 30 | 40 |
��ѹǿ/100kPa | 1.0 | 1.2 | 1.3 | 1.4 | 1.4 |
��ƽ��ʱ�����������������ʵ���Ϊ________mol��H2O��ת����Ϊ________��
�����¶��·�Ӧ��ƽ���ѹ����Kp��________kPa(�������2λ��Ч����)��
(3)����25��������㶨��1L������Ͷ����������̿��������壬�������淴ӦC��H2O(g)CO��H2���ѽ���ƽ�⣬��40 minʱ�ٳ���һ����H2��50minʱ�ٴδﵽƽ�⣬��Ӧ�����и����ʵ�Ũ����ʱ��仯��ͼ��ʾ��
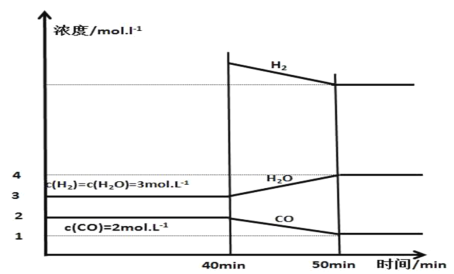
��40minʱ���ٳ����H2�����ʵ���Ϊ________mol��
��40��50 min��H2��ƽ����Ӧ����Ϊ________mol��L��1��min��1��
(4)���͵������������ڽ����ơ�������Ͷ�����(Na2Sx)�ֱ���Ϊ�����缫�ķ�Ӧ�����Al2O3�մ�(�ɴ���Na��)Ϊ����ʣ���ԭ����ͼ��ʾ��
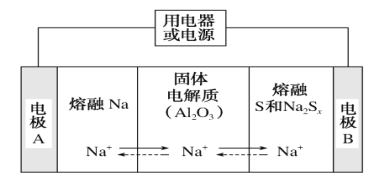
���ŵ�ʱ���缫AΪ________����S����________��Ӧ�����������ԭ������
�����ʱ���ܷ�ӦΪNa2Sx=2Na��Sx(3<x<5)��Na���ڵ缫��ֱ����Դ________�������������ĵ缫��ӦʽΪ_________��
���𰸡���131.3 kJ��mol��1 1.4 40% 27 6 0.1 �� ��ԭ �� Sx2--2e-=Sx
��������
(1)��֪̼(ʯī)��H2��CO��ȼ���ȷֱ�Ϊ393.5kJ��mol��1��285.8kJ��mol��1��283kJ��mol��1����
��C(s)��O2(g)��CO2(g)����H����393.5kJ��mol��1
��![]() O2(g)��H2(g)��H2O(l)����H����285.8kJ��mol��1
O2(g)��H2(g)��H2O(l)����H����285.8kJ��mol��1
��CO(g)��![]() O2(g)��CO2(g)����H����283kJ��mol��1
O2(g)��CO2(g)����H����283kJ��mol��1
��H2O(l)=H2O(g)����H����44kJ��mol��1
���ݸ�˹���ɿ�֪�����������������õ�C(s)��H2O(g)![]() CO(g)��H2(g)����H����131.3 kJ��mol��1��
CO(g)��H2(g)����H����131.3 kJ��mol��1��
(2)���ݷ���ʽ��֪
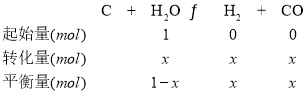
ѹǿ֮�������ʵ���֮�ȣ���(1��x+x+x):1��1.4:1�����x��0.4��
���������Ϸ�����֪ƽ��ʱ�����������������ʵ���Ϊ1.4mol��H2O��ת����Ϊ40%��
�����¶��·�Ӧ��ƽ���ѹ����Kp��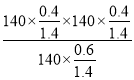 ��27kPa��
��27kPa��
(3)����50minʱH2�����ʵ���Ϊx mol���¶Ȳ���ƽ�ⳣ�����䣬�����ݻ���1L�������ͼ���֪ƽ�ⳣ��K=![]() �����x��8������CO�ı仯����1mol������40minʱ�ٳ����H2�����ʵ���Ϊ8mol+1mol��3mol��6mol��
�����x��8������CO�ı仯����1mol������40minʱ�ٳ����H2�����ʵ���Ϊ8mol+1mol��3mol��6mol��
�����������ı仯����1mol������40��50 min��H2��ƽ����Ӧ����Ϊ![]() ��0.1mol��L��1��min��1��
��0.1mol��L��1��min��1��
(4)���ŵ�ʱ��ʧȥ���ӣ���缫AΪ�������缫B����������S������ԭ��Ӧ��
���ŵ�ʱ��ʧȥ���ӣ���缫AΪ���������ʱ��Na���ڵ缫����������ֱ����Դ������������������ʧȥ���ӵ�������Ӧ��������ܷ�ӦΪNa2Sx=2Na��Sx(3��x��5)��֪�������缫��ӦʽΪSx2--2e-=Sx��



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����ij�¶ȣ�T�棩��ˮ��Һ�У�c(H+)��10xmol/L��c(OH��)��10ymol/L��x��y��ϵ��ͼ��ʾ��
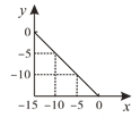
��1�����¶��£�ˮ�����ӻ�Ϊ________��T��_____25�棨������������С������������������
��2�������¶���pH��11��NaOH��Һ��pH��1��HCl��Һ�������ϣ���Ϻ���Һ��pHԼΪ________������֪lg2��0.3��
��3���ڴ��¶��£���pH��13��NaOH��ҺVaL��pH��1��������ҺVbL��ϡ������û��Һ��pH��2����Va:Vb ��_______��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����ͼ��һ��ʵ������ȡ��ϩ����֤��ϩ���л�ԭ�Ե�ʵ��װ�á�
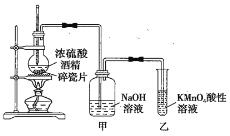
��ش��������⡣
��1����ƿ�����Ƭ��������__��װ���е����Դ�����__��
��2��ʵ�鲽�裺��_�����ڸ�װ����ע����Ӧ���Լ�����ͼ��ʾ������__��ʵ��ʱ����ƿ��Һ��������ڡ�
��3���ܹ�˵����ϩ���л�ԭ�Ե�������__��װ�ü�������__������װ�ã��Ƿ�Ҳ�ܼ�����ϩ���л�ԭ�ԣ�_���������������������������������ɣ�__��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��X��Y��Z��W��R��QΪǰ30��Ԫ�أ���ԭ��������������X��Ԫ�����ڱ���ԭ�Ӱ뾶��С��Ԫ�أ�YԪ�ػ�̬ԭ���������ܼ����е��ӣ���ÿ���ܼ��ϵĵ�������ȣ�ZԪ�ػ�̬ԭ�ӵĵ���������ͬ����Ԫ�ػ�̬ԭ������ࣻW��Zͬ���ڣ���һ�����ܱ�Z�ĵͣ�R��Yͬ���壻Q�������ֻ��һ�����ӣ��������Ӳ����2n2�����ӣ�n��ʾ���Ӳ�����������ش��������⣺
��1��Q�ĺ�������Ų�ʽΪ___��
��2��������X2W2��W���ӻ���ʽΪ___��ZW2-�Ŀռ乹��Ϊ___��
��3��Y��R������������зе�ϸߵ���___���ѧʽ����ԭ����___��
��4��Y�ж���ͬ�������壬����һ��ͬ��������ľ����ṹ��ͼ��ʾ���þ���ľ���������Yԭ�ӵĸ���Ϊ___��Yԭ�ӵ���λ��Ϊ___���������ı߳�Ϊapm��������ܶ�Ϊ��g/cm3�����ӵ�������ֵNAΪ___���ú�a�����Ĵ���ʽ��ʾ����
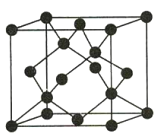
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ij����Ϣ�صĽṹ��ʽΪCH3(CH2)5CH=CH(CH2)9CHO��Ϊ��֤�����ʼȺ���ȩ���ֺ���̼̼˫����ijͬѧ��������¼��ַ������Կ��ܲ��������������Ԥ�⡣
a.��������м�����ˮ���۲쵽��ˮ��ɫ��Ȼ���ټ���������Һ��ˮԡ���ȣ��۲쵽�������γ�
b.��������м���������Һ��ˮԡ���ȣ��۲쵽�������γɣ�Ȼ���ټ�����ˮ���۲쵽��ˮ��ɫ
c.��������еμ����CCl4��Һ���۲쵽��Һ��ɫ��Ȼ���ټ���������Һ��ˮԡ���ȣ��۲��������γ�
(1)����Ϊ�����������в�ͨ����_____(�����)��������_________________________��
(2)�ֱ�д�����CCl4��Һ��������Һ����л��ﷴӦ�Ļ�ѧ����ʽ:________��__________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���ҹ��ڶ�������֤���õ��Ǿ�����ɫ�������ܵ�PETG�²��ϣ��ò��Ͽ��Ի��������ã����Ҷ��ܱ����������κ���Ⱦ��PETG�Ľṹ��ʽ���£�

�ɲ��õĺϳ�·����ͼ��ʾ��
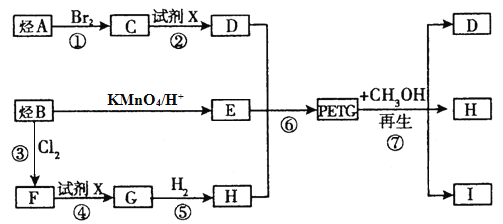
��֪����A�IJ����Ǻ���һ������ʯ�ͻ�����չˮƽ����Ҫ��־֮һ��
��
![]()
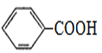
��RCOORl��R2OH��RCOOR2��R1OH(R��R1��R2��ʾ����)
�Իش��������⣺
(1)C������Ϊ________����Ӧ��������Ϊ_______��
(2)��Ӧ����������Ϊ__________���Լ�XΪ________��
(3)д��I�Ľṹ��ʽ��___________��
(4)д����Ӧ���Ļ�ѧ����ʽ��____________
(5)��E��Ϊͬ���칹�壬�����������������л��ﹲ��_____�֣����к˴Ź���������4��壬�������Ϊ1:2:2:1��һ��ͬ���칹��Ľṹ��ʽΪ_______��
�����㻯�����һ���������ܷ���������Ӧ��������NaHCO3��Һ��Ӧ�������塣
(6)���Լױ�Ϊԭ�ϣ�����Ʊ������ᱽ�����ĺϳ�·��_____________��(���Լ���ѡ���ϳ�·��ʾ���������)
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ij�ֻ������ͼ���� W��X��Y��Z ���ֶ�����Ԫ����ɣ����� W��Y��Z �ֱ�λ��������ͬ���ڣ�Y ���������������� W ���������������Ķ�����W��X��Y ���ּ����ӵĺ�������Ų���ͬ������˵������ȷ����
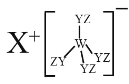
A.ԭ�Ӱ뾶��W < X < Y < Z
B.X �� Y��Y �� Z �����γɾ���Ư���ԵĻ�����
C.�����ӵ������ԣ� W X
D.W �� X ������������ˮ��������Ӧ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�����и��ݷ�Ӧԭ����Ƶ�Ӧ�ã�����ȷ���ǣ� ��
A.CO32-��H2O![]() HCO3-��OH�� �ȵĴ�����Һ��ϴ����
HCO3-��OH�� �ȵĴ�����Һ��ϴ����
B.Al3����3H2O![]() Al(OH)3��3H�� ������ˮ
Al(OH)3��3H�� ������ˮ
C.TiCl4��(x��2)H2O(����)![]() TiO2��xH2O����4HCl �Ʊ�TiO2��xH2O
TiO2��xH2O����4HCl �Ʊ�TiO2��xH2O
D.SnCl2��H2O![]() Sn(OH)Cl��HCl �����Ȼ�������Һʱ������������
Sn(OH)Cl��HCl �����Ȼ�������Һʱ������������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����1��ij����ֻ��C��H��O����Ԫ�أ������ģ����ͼ��ʾ�������й���12��ԭ�ӡ�
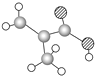
�����ʵĽṹ��ʽΪ_______________�������������������ŵ�����Ϊ______________________��
��2�����и������ʣ� �� O2��O3 �� H2��D2��T2 �� 12C��14C �� CH3CH2CH2CH3 �� (CH3)2CH2CH3 ������Ͷ��� ��CH3CH2CH2CH��C2H5��CH3��CH3CH2CH2CH��CH3��C2H5 ��Ϊͬϵ�����__________�� ��Ϊͬ���칹�����________����Ϊͬλ�ص���________�� ��Ϊͬ�����������_________����ͬһ���ʵ���__________��
��3����̪�dz��õ����ָʾ������ṹ��ʽ��ͼ��ʾ��
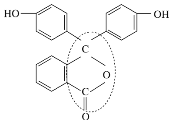
�ٷ�̪�ķ���ʽΪ___________________________________��
�ڴӽṹ�Ϸ�����̪�ɿ���_____________________��
A��ϩ�������������� B�����㻯����
C���������� D����������
E���������� F����������
�۷�̪�ṹ��ʽ�л����ߵĵط����Ѽ���_________________��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com