
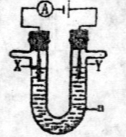
��15�֣���1�����ԭ���ڻ�ѧ��ҵ���й㷺��Ӧ�á��ֽ�����Ƶ�ԭ���ͨ��������ͼ�е�������������aΪ���Һ��X��Y������缫�⣬��
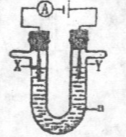
����X��Y��Ϊ���Ե缫��aΪ����ʳ��ˮ������ʱ����Y�缫��Ӧ����ķ����� ��
����X��Y�ֱ�Ϊʯī������a��Ϊ���͵�NaCl��Һ����������л����ɰ�ɫ���壬�������ܷ�Ӧ��ѧ����ʽΪ
��
�ð�ɫ����¶���ڿ����У��ɹ۲쵽��������
��
����X��Y��Ϊ���Ե缫��aΪһ��Ũ�ȵ�����ͭ��Һ��ͨ��������ܷ�Ӧ��ѧ����ʽΪ ��ͨ��һ��ʱ�����������Һ�м���0��05 mol Cu(OH)2��ǡ�ûָ����ǰ��Ũ�Ⱥ�pH����������е���ת�Ƶ����ʵ���Ϊ mol��
��2�����ù�ҵ�����ӽ���Ĥ�����ռ��ԭ��������ͼ��ʾװ�õ��K2SO4��Һ��
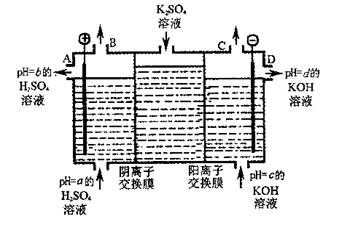
�ٸõ��۵�������ӦʽΪ ��ͨ�������ӽ���Ĥ�������� ���>������<����=����ͨ�������ӽ���Ĥ����������
��ͼ��a��b��c��d�ֱ��ʾ�й���Һ��pH����a��b��c��d��С�����˳��Ϊ ��
�۵��һ��ʱ���B����C�ڲ��������������Ϊ ��

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
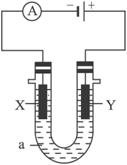
��1����X��Y���Ƕ��Ե缫��a�DZ���NaCl��Һ��ʵ�鿪ʼʱ��ͬʱ�����߸����뼸�η�̪��Һ����
�ٵ�����X���ϵĵ缫��ӦʽΪ________________����X�������۲쵽��������______
_______________________��
��Y�缫�ϵĵ缫��ӦʽΪ________________________������õ缫��Ӧ����ķ�����___
______________________��
��2������õ�ⷽ��������ͭ�����Һaѡ��CuSO4��Һ����
��X�缫�IJ�����________________________���缫��ӦʽΪ______________________��
��Y�缫�IJ�����________________________���缫��ӦʽΪ_______________________��
��˵�������ʷ����ĵ缫��Ӧ����д����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��15�֣���1�����ԭ���ڻ�ѧ��ҵ���й㷺��Ӧ�á��ֽ�����Ƶ�ԭ���ͨ��������ͼ�е�������������aΪ���Һ��X��Y������缫�⣬��
����X��Y��Ϊ���Ե缫��aΪ����ʳ��ˮ������ʱ����Y�缫��Ӧ����ķ����� ��
����X��Y�ֱ�Ϊʯī������a��Ϊ���͵�NaCl��Һ����������л����ɰ�ɫ���壬�������ܷ�Ӧ��ѧ����ʽΪ
��
�ð�ɫ����¶���ڿ����У��ɹ۲쵽��������
��
����X��Y��Ϊ���Ե缫��aΪһ��Ũ�ȵ�����ͭ��Һ��ͨ��������ܷ�Ӧ��ѧ����ʽΪ ��ͨ��һ��ʱ�����������Һ�м���0��05 molCu(OH)2��ǡ�ûָ����ǰ��Ũ�Ⱥ�pH����������е���ת�Ƶ����ʵ���Ϊ mol��
��2�����ù�ҵ�����ӽ���Ĥ�����ռ��ԭ��������ͼ��ʾװ�õ��K2SO4��Һ��
�ٸõ��۵�������ӦʽΪ ��ͨ�������ӽ���Ĥ�������� ���>������<����=����ͨ�������ӽ���Ĥ����������
��ͼ��a��b��c��d�ֱ��ʾ�й���Һ��pH����a��b��c��d��С�����˳��Ϊ ��
�۵��һ��ʱ���B����C�ڲ��������������Ϊ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
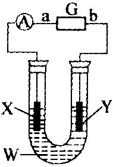
(1)���õ��ԭ������ͼװ������ɴ�ͭ�ᴿ����������ҺWΪ_________����������XΪ_________��
(2)��ֱ֪����ԴGΪ������أ����������һ�����Ϳɳ���أ�����ͨ���ܵ����ȣ��õ���ܳ�ʱ�䱣���ȶ��ķŵ��ѹ��������ص��ܷ�ӦʽΪ��
3Zn+2K2FeO4+8H2O![]() 3Zn(OH)2+2Fe(OH)3+4KOH��b�缫��ӦʽΪ_________���ŵ�ʱÿת��3 mol���ӣ�������_________mol_________����ԭ��
3Zn(OH)2+2Fe(OH)3+4KOH��b�缫��ӦʽΪ_________���ŵ�ʱÿת��3 mol���ӣ�������_________mol_________����ԭ��
(3)��X��XΪʯī�壬WΪCuSO4��Һ�����һ��ʱ��������IJ���Һ�м����������۳�ַ�Ӧ�����ˡ����ɡ����أ�������������3.2g��ϴ������ɡ����أ�����Y������1.6 g����������Ͻ����������и�����ص�п�缫��������_________g��������ҺpHΪ_________��ԭCuSO4��Һ�����ʵ���Ũ��Ϊ_________mol��L-1��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��1�����ԭ���ڻ�ѧ��ҵ���й㷺��Ӧ�á��ֽ�����Ƶ�ԭ���ͨ��������ͼ�е�������������aΪ���Һ��X��Y������缫�⣬��
����X��Y��Ϊ���Ե缫��aΪ����ʳ��ˮ������ʱ����Y�缫��Ӧ����ķ����� ��
����X��Y�ֱ�Ϊʯī������a��Ϊ���͵�NaCl��Һ����������л����ɰ�ɫ���壬�������ܷ�Ӧ��ѧ����ʽΪ
��
�ð�ɫ����¶���ڿ����У��ɹ۲쵽��������
��
����X��Y��Ϊ���Ե缫��aΪһ��Ũ�ȵ�����ͭ��Һ��ͨ��������ܷ�Ӧ��ѧ����ʽΪ ��ͨ��һ��ʱ�����������Һ�м���0��05 mol Cu(OH)2��ǡ�ûָ����ǰ��Ũ�Ⱥ�pH����������е���ת�Ƶ����ʵ���Ϊ mol��������������Դ*��
��2�����ù�ҵ�����ӽ���Ĥ�����ռ��ԭ��������ͼ��ʾװ�õ��K2SO??4��Һ��
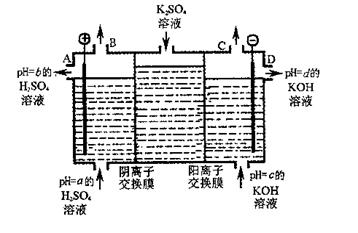
�ٸõ��۵�������ӦʽΪ ��ͨ�������ӽ���Ĥ�������� ���>������<����=����ͨ�������ӽ���Ĥ����������
��ͼ��a��b��c��d�ֱ��ʾ�й���Һ��pH����a��b��c��d��С�����˳��Ϊ ��
�۵��һ��ʱ���B����C�ڲ��������������Ϊ ��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com