
1 1 |
3 1 |
 ����
����1 1 |
3 1 |
 ����ͬ���칹�壬�ʴ�Ϊ���ܢݣ�
����ͬ���칹�壬�ʴ�Ϊ���ܢݣ�


| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A����ȥ�����Ȼ��ƹ����е��Ȼ�炙��ʵ�����ü��������ķ��� | B����FeCl2��Һ�к���I-���ʣ����ù�����Cl2�����������ȡ��Һ | C���Ӻ�ˮ����ȡ�壬һ������Ũ����ĺ�ˮ��ͨ��Cl2���ٹ����ȿ�����ˮ�������������� | D���������Na2CO3��NaHCO3�����Էֱ������Һ���ټ������ʯ��ˮ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
I.���������ʽ��з��ࣺ
��![]() H��
H��![]() H �� ��O2��O3 ���� CH2=CHCH3 ��CH2=C(CH3)2
H �� ��O2��O3 ���� CH2=CHCH3 ��CH2=C(CH3)2
���Ҵ���C2H5OH������ѣ�CH3��O��CH3�� ��
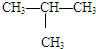
�������飨CH3CH2 CH2 CH3�����춡�飨 �� ��
�� ��
���飨CH4������飨C3H8������C6H5OH ��C6H5CH2OH(C6H5-Ϊ����)
��1����Ϊͬλ�ص��� ������š���ͬ����
��2����Ϊͬϵ����� ��
��3����Ϊͬ���칹����� ��
(4) ��Ϊͬ����������� .
II.���ڢٱ� �ڱ�ϩ �ۼױ� �ܱ��� �ݱ����У�������š���ͬ��
��1���ܺͽ����Ʒ�Ӧ�ų�H2������
��2������NaOH��Һ��Ӧ����
��3������������Ũ��ˮ��Ӧ����
��4������ԭ�� һ������ͬһƽ�����
��5����ʹ����KMnO4��Һ��ɫ����
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com