
�ٳ�ȡa(g)���ᾧ�����100.00mLˮ��Һ��
��ȡ25.00mL�IJ�����Һ������ƿ�У���������ϡH2SO4����Ũ��Ϊb(mol��L-1)KMnO4��Һ�ζ����������ķ�ӦΪ��3H2SO4+2KMnO4+5H2C2O4�T�TK2SO4+2MnSO4+8H2O+10CO2��
��1��Ϊ��֤ʵ��ľ�ȷ�ȣ�ʵ����У�Ӧ������������ѡȡһ��������________��
a��������ƽ��b��50mL��ʽ�ζ��ܣ�c��100mL��Ͳ��d��100mL����ƿ��e���ձ���f����ͷ�ιܣ�g����ƿ��h����������i��ҩ�ף�j��50mL��ʽ�ζ���
��2��ʵ����У��ζ�ʱ��KMnO4��ҺӦװ��________ʽ�ζ����У�
��3���ڵζ������У�����________������________���۾�ע��________���ﵽ�յ�ı�־��________��
��4����ʵ������У�����������ˮ��ϴ��ƿ�ڱڣ��������ζ���������õ�xֵ__________���ƫ����ƫС�����䡱����
��5�����ζ������У�����ȥb(mol��L-1)KMnO4c(mL)���������ƵIJ�����Һ�����ʵ���Ũ��Ϊ________���ɴ˿ɼ�������ᾧ���нᾧˮ��x
��4�����䣨5��0.1bc(mol/L) ����NaOH��Һ�������CO2��Ӧ������Na2CO3����H2SO4�ζ�Na2CO3��Һ��Ӧ�������ν��� ��H++ ��ѡ�ü�����ָʾ���������ɫ��Χ��pH=3.1��4.4���ζ���Ӧ���ڽ��У��ɹ�ϵʽ2NaOH ע�⣺�к͵ζ������У�ѡ�ò�ͬ��ָʾ�����ζ��յ��pHҲ���ܲ�ͬ�����·�����ͬ�ķ�Ӧ��
��������1��a��d��e��f��h��i ��2���� ��3������ƿ����˳ʱ�뷽��Բ���˶������Ƶζ��ܣ���ƿ����Һ��ɫ�仯��ָʾ����ɫ��������ڲ���ȥ
![]()
![]() =
=![]() ����ʱ��Һ�������ԣ���H++
����ʱ��Һ�������ԣ���H++![]() =CO2��+H2O����Һ�������ԣ�
=CO2��+H2O����Һ�������ԣ�![]() ֪�����ĵ�H2SO4�������䣬��VA=VB����ѡ�÷�̪��ָʾ������̪�ı�ɫ��ΧΪpH=8��10���ζ����ٽ��У�����H2SO4�������٣���VA<VB��
֪�����ĵ�H2SO4�������䣬��VA=VB����ѡ�÷�̪��ָʾ������̪�ı�ɫ��ΧΪpH=8��10���ζ����ٽ��У�����H2SO4�������٣���VA<VB��


 Ӧ�����������Ĵ���ѧ������ϵ�д�
Ӧ�����������Ĵ���ѧ������ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| 50m |
| 9c?(b-a) |
| 50m |
| 9c?(b-a) |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�ٳ�ȡW g�����ᾧ�壬�������Ƴ�100.0 mLˮ��ҺΪ����Һ��
��ȡ25.0 mL����Һ������ƿ�У��ټ���������ϡH2SO4��
����Ũ��Ϊa mol��L��1��KMnO4����Һ���еζ����ζ�ʱ�����ķ�ӦΪ��
2KMnO4+5H2C2O4+3H2SO4===K2SO4+2MnSO4+10CO2��+8H2O
��ش�
(1)�ζ�ʱ����KMnO4��Һװ��_________�ζ����У�����ʱ��_________������ƿ��
(2)���ζ�ʱ���õ�KMnO4��Һ����ö�����Ũ�ȱ�С�����ɴ˲�õ�xֵ��_________(�ƫ��ƫС�����䡱)��
(3)����ζ��յ�ʱ����ȥV mL KMnO4��Һ������������Һ�����ʵ���Ũ��Ϊ_________mol��L��1��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2015�켪��ʡ�߶���ѧ����ĩ���ƻ�ѧ�Ծ��������棩 ���ͣ�ʵ����
���ᾧ�����ɿɱ�ʾΪH2C2O4��xH2O��Ϊ�ⶨxֵ����������ʵ�顣
�ٳ�ȡm g���ᾧ�壬���100��0 mL��Һ��
��ȡ25��0 mL���������Һ������ƿ�У���������ϡH2SO4����Ũ��Ϊc mol��L��1 KMnO4��Һ�ζ����ζ�ʱ����������ӦΪ��2KMnO4��5H2C2O4��3H2SO4=K2SO4��10CO2����2MnSO4��8H2O
��ش��������⣺
��1��ʵ�����Ϊ������ȷŨ�ȵIJ�����Һ������Ҫ��ʵ��������Ҫ�У���ƽ(������)���ձ���ҩ��____________��____________��____________��
��2����ʵ����У��ζ�ʱKMnO4��ҺӦװ��________ʽ�ζ����У���ƿ��________
(���Ҫ���� ������Ҫ��)�μ�ָʾ����
��3���ڵζ������У�Ŀ��Ӧע��______________________��
��4�����ζ�ʱ���ζ�ǰ�����ζ����ֱ�ΪamL��bmL����˼����xֵΪ________��
��5������ȡ����aʱ���ӣ���ȡ����bʱ���ӣ�������xֵ________(�ƫ����ƫ С�����䡱)��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2013������и߶���ѧ����ĩ���Ի�ѧ�Ծ� ���ͣ�ʵ����
(5��)���ᾧ�����ɿɱ�ʾΪH2C2O4��xH2O��Ϊ�ⶨxֵ����������ʵ�飺
�ٳ�ȡW g�����ᾧ�壬�������Ƴ�100.0 mLˮ��ҺΪ����Һ��
��ȡ25.00 mL����Һ������ƿ�У��ټ���������ϡH2SO4
����Ũ��Ϊa mol•L-1��KMnO4����Һ���еζ����ζ�ʱ�����ķ�ӦΪ��
2KMnO4+5H2C2O4+3H2SO4=====K2SO4+2MnSO4+l0CO2
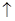 +8H2O
+8H2O
��ش�
(1)�ζ�ʱ����KMnO4��Һװ�� �ζ����У�����ʱ�� ������ƿ��
(2)���ζ�ʱ���õ�KMnO4��Һ����ö�����Ũ�ȱ�С�����ɴ˲�õ�xֵ�� (�ƫ��ƫС�����䡯��)��
(3)����ζ��յ�ʱ����ȥV mL KMnO4��Һ������������Һ�����ʵ���Ũ��Ϊ mol•L-1��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com