
��22�֣���֪X��Y��Z���ֶ���������Ԫ����Ԫ�����ڱ��е�λ�ù�ϵ��ͼ��ʾ��
| Z | |
| Y | X |
��1�� Y 2��
��2����ÿ�ո��2�֣� H2SO4 < HClO4 HF
HF���� ������ H��O��Cl
������ H��O��Cl
��3����������IIIA�� 2�� 2Al + 2OH��+2H2O = 2AlO2 ��+ 3H2�� 2��
ȡ��״��С��ͬ��þƬ����Ƭ�ֱ��������ͬ��Ũ����ͬ��ϡ���ᷴӦ���۲�������ݵĿ��� ��3�֣�������2�֣��۲�ָ��1�֡����غŲ���ȱһ����1�֡��ý������ˮ��Ӧ����Ũ������Һ���ԱȽϵȺ����������÷֣�
��4���� 2�� 3C + SiO2 =" " 2CO �� + SiC 3��
����



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

��1��д��Ԫ�ط��ţ�X___________��Y___________��Z___________��
��2��д���٢ڢܵ����ӷ�Ӧ����ʽ��
��__________________________________________________________��
��__________________________________________________________��
��__________________________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
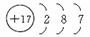
��ش��������⣺
��1��д��Z��ԭ�ӽṹʾ��ͼ____________________________��
��2��д��ͼ��D�ĵ���ʽ____________________________��
��3��ȷ��ͼ�е���F��������____________________________��
��4������ͼ�з�Ӧ���ڱ�״��������
��5��д��ͼ��G��Һ��H��Һ���Ϸ�����Ӧ�����ӷ���ʽ__________________________��
��6������ͼ��ʾװ�ã�����������ʢ��A������Һ������Ϊ���ʯī�缫���������ֱ�ͨ��X2��Z2���壬��������ָ�뷢��ƫת��ͨ��X2����ĵ缫������_________________����д������������������ͨ��Z2����ĵ缫��ӦʽΪ____________________________��
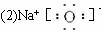
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

��ش��������⣺
(1)д��Z��ԭ�ӽṹʾ��ͼ_______________��
(2)д��ͼ��D�ĵ���ʽ_______________��
(3)ȷ��ͼ�е���F��������_______________��
(4)����ͼ�з�Ӧ���ڱ�״��������
(5)д��ͼ��G��Һ��H��Һ���Ϸ�����Ӧ�����ӷ���ʽ_______________��
(6)����ͼ��ʾװ�ã�����������ʢ��A������Һ������Ϊ���ʯī�缫���������ֱ�ͨ��X2��Z2���壬��������ָ�뷢��ƫת��ͨ��X2����ĵ缫������_______________(��д��������������)��ͨ��Z2����ĵ缫��ӦʽΪ_______________��

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��22�֣���֪X��Y��Z���ֶ���������Ԫ����Ԫ�����ڱ��е�λ�ù�ϵ��ͼ��ʾ��
| Z |
|
| Y | X |
��ش𣺣�1������Ԫ����ԭ�Ӱ뾶������ ����X��Y��Z��
��2����X�ĵ����ǻ���ɫ���壬��X��Y������������Ӧ��ˮ���������ǿ��˳��Ϊ ���û�ѧʽ��ʾ������Xͬ����Ԫ���⻯�����۷е���ߵ��� ���û�ѧʽ��ʾ����ԭ���� ��X��������ˮ������֮һ����Ư���ԣ�д�������ʽṹʽ ��
��3����X�ĵ�����ǿ�ᡢǿ����Һ��Ӧ���ܷų���������Ԫ��X�����ڱ��е�λ�� д��X���ʺ��ռ���Һ��Ӧ�����ӷ���ʽ
̽��X��Y����Ԫ�ؽ�����ǿ����ʵ�鷽���� ��
��4����Y����������������ά�IJ��ϣ���YԪ�ص������� ��
��ҵ�����õ���Z��Y������������·�Ӧ������һ�־��пռ�������״�ṹ�Ļ������һ�����������ж����壬д����Ӧ��ѧ����ʽ ��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com