
����ʵ���У�����ʵ����������ó��Ľ��۲���ȷ���� �� ��
�� �� | �� �� | �� �� | |
A | ȡ���õ�Na2O2��ĩ�������еμӹ��������� | ������ɫ���� | Na2O2û�б��� |
B | ��һƬ�������ھƾ������������� | �����ۻ��������� | ��������������Al2O3��Ĥ����Al2O3�۵����Al |
C | ��ɫ��Һ�еμ���ˮ��CCl4�������� | �²���Һ����ɫ | ԭ��Һ����I�� |
D | ����ҺX�м���ϡ���ᣬ������������ɫ��ζ����ͨ�����ʯ��ˮ�� | ���ɰ�ɫ���� | ��ҺX�п��ܺ��� CO32���� HCO3�� |
A. A B. B C. C D. D

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ��2017���Ĵ�ʡ�ɶ��и����ڶ���ģ�⿼�����ۻ�ѧ�Ծ��������棩 ���ͣ�ѡ����
����ѡ���У��������ʵ�����ģ������������ޣ��ܹ������Ӧʵ�����
ѡ�� | ʵ������ | ��Ӧʵ�� |
A | �Թܡ�����̨������ | �����������Ʊ� |
B | ��ƿ��©����˫���������ܡ��������ľ����ҩ�� | ����MnO2��H2O2�ֽ����ʵ�Ӱ�� |
C | 500mL����ƿ���ձ�������������ƽ | ���� 500mL1.00mol/LNaCl��Һ |
D | ���żܡ��������ƾ��ơ�����ǯ | ���ڿ�����ȼ�� |
A. A B. B C. C D. D
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2016-2017ѧ��ɽ��ʡ�����и߶���ѧ�ڵ�һ�ν��Բ��Ի�ѧ�Ծ��������棩 ���ͣ������
�±�ΪԪ�����ڱ�ǰ�����ڵ�һ���֡�
X | Z | ||
W | Y | R | |
��1��X���⻯��ķе���W���⻯��Ƚϣ��е�ϸߵ��ǣ�______________���ѧʽ����ԭ����_____________________��
��2��������ѡ����ѡ��X�Ļ�̬ԭ�ӵ������ԭ�ӹ����ʾʽ____________����һԭ�ӹ����ʾʽ������Ϊ��̬ԭ�ӵĹ����ʾʽ����Ϊ��������____________��������ţ���
A.  B.
B. 
C������ԭ�� D�����ع���
��3������W��Y��RԪ��ԭ��ʧȥ�����һ��������Ҫ�����ɶൽ�ٵ�˳��Ϊ��_______________����Ԫ�ط��ţ���
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2016-2017ѧ��ɽ��ʡ�����и߶���ѧ�ڵ�һ�ν��Բ��Ի�ѧ�Ծ��������棩 ���ͣ�ѡ����
���е��Ӳ��У�������f�ܼ����ǣ� ��
A. K���Ӳ�
B. L���Ӳ�
C. M���Ӳ�
D. N���Ӳ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2016-2017ѧ���Ĵ�ʡ�ɶ������и�һ��ѧ����ĩ��⻯ѧ�Ծ��������棩 ���ͣ�ʵ����
Ϊ��̽��ľ̿��ŨH2SO4��Ӧ�IJ������Ƿ����CO2��ijͬѧѡ����ͼ��ʾװ�ý���ʵ�飺
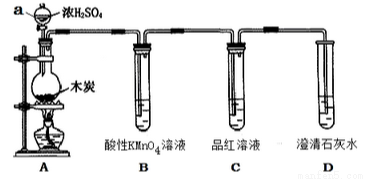
��1������a������Ϊ��__________������װ������ʵ��װ�ú���װ��ҩƷ֮ǰ�����ʵ�������________________��
��2��д��A�з�����Ӧ�Ļ�ѧ����ʽ____________________����װ�û���������ʵ������ȡCl2����д��ʵ������ȡCl2�����ӷ���ʽ���õ����ű�������ת��________________��
��3��װ��B��������____________________��
��4����˵��������һ������CO2�����ʵ������Ϊ________________��
��5����������Ϊ98����Ũ���ᣬ���ܶ�Ϊ1.84g/ml����Ũ��������ʵ���Ũ��Ϊ__________���ø���������500ml 0.5mol/L��ϡ���ᣬ��Ҫ��������Ͳ���ձ�������������ͷ�ι��⣬����Ҫ____________������ʱ���ڸ��ӿ̶��ߣ����Ũ�Ȳ�����Ӱ��Ϊ___________ ���ƫ��ƫС������Ӱ�족����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2016-2017ѧ���Ĵ�ʡ�ɶ������и�һ��ѧ����ĩ��⻯ѧ�Ծ��������棩 ���ͣ�ѡ����
�������ӷ���ʽ��д��ȷ���ǣ� ��
A������ˮ�ķ�Ӧ Na+ �� H2O= OH��+ H2��
B��NaHCO3��Һ������ķ�Ӧ CO32- ��2H+��H2O + CO2��
C����FeCl3��Һ��ʴͭ��·�壺 Cu + 2Fe3+ �� Cu2+ + 2Fe2+
D��AlCl3��Һ�м��������ˮ Al3+��4OH����AlO2����2H2O
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2016-2017ѧ���Ĵ�ʡ�ɶ������и�һ��ѧ����ĩ��⻯ѧ�Ծ��������棩 ���ͣ�ѡ����
����������ȷ���� �� ��
A. NO��Ħ������Ϊ30g
B. ���³�ѹ�£�14gһ����̼��ռ���Ϊ11.2 L
C. �����ʵ�����Na2O2 ��Na2O���������������������
D. �谢���ӵ�����ΪNA�����³�ѹ��O2��O3�Ļ����32g�����з�����ΪNA
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2016-2017ѧ������и߶�3��ѧ��ѧҵ�������л�ѧ�Ծ��������棩 ���ͣ�ѡ����
����ʽΪC5H7Cl���л����ṹ��������
A��ֻ��һ��˫����ֱ���л��� B����������˫����ֱ���л���
C������һ��˫���Ļ�״�л��� D������һ��������ֱ���л���
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2017���㽭ʡ�����и���3�½�ѧ�������⿼�Ի�ѧ�Ծ��������棩 ���ͣ�ѡ����
�������ʵ�ˮ��Һ��ˮ����ʼ��Ե��ǣ� ��
A. NaOH B. NH4Cl C. CH3COONa D. HC1
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com