


 ̽���빮�̺��Ͽ�ѧ����������ϵ�д�
̽���빮�̺��Ͽ�ѧ����������ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
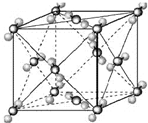 ��2009?��Ǩģ�⣩��֪A��B��C��D��E�������ڱ���ǰ�����ڵ�Ԫ�أ����ǵĺ˵����A��B��C��D��E������B��D��Eԭ��������Ӳ��p�ܼ���������ϵĵ��Ӵ��ڰ���״̬��ͨ������£�A��һ�����������Ϊ�Ǽ��Է��ӣ��侧���ṹ������ͼ��ʾ��ԭ������Ϊ31��Ԫ���أ�Ga����Ԫ��B�γɵ�һ�ֻ������Ǽ���C����Ϊ�����ĵ�һ���뵼����Ϻ�GaEΪ�����ĵڶ����뵼�����֮���ڽ�10��Ѹ�ٷ�չ�����ĵ��������Ͱ뵼����ϣ�
��2009?��Ǩģ�⣩��֪A��B��C��D��E�������ڱ���ǰ�����ڵ�Ԫ�أ����ǵĺ˵����A��B��C��D��E������B��D��Eԭ��������Ӳ��p�ܼ���������ϵĵ��Ӵ��ڰ���״̬��ͨ������£�A��һ�����������Ϊ�Ǽ��Է��ӣ��侧���ṹ������ͼ��ʾ��ԭ������Ϊ31��Ԫ���أ�Ga����Ԫ��B�γɵ�һ�ֻ������Ǽ���C����Ϊ�����ĵ�һ���뵼����Ϻ�GaEΪ�����ĵڶ����뵼�����֮���ڽ�10��Ѹ�ٷ�չ�����ĵ��������Ͱ뵼����ϣ��鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com