
����Ŀ������������(FexNy)�ڴż�¼�����������Ź㷺��Ӧ��ǰ����ijFexNy���Ʊ���������������ͪ���Ҵ����롣
��1��Fe3����̬��������Ų�ʽΪ___��
��2����ͪ(CH3CCH3O)������̼ԭ�ӹ�����ӻ�������__��1mol��ͪ�����к�����������ĿΪ___��
��3��C��H��O����Ԫ�صĵ縺����С�����˳��Ϊ____��
��4���Ҵ��ķе���ڱ�ͪ��������Ϊ______��
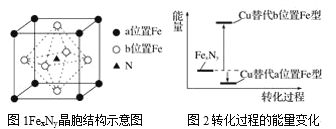
��5��ijFexNy�ľ�����ͼ1��ʾ��Cu������ȫ����þ�����aλ��Fe����bλ��Fe���γ�Cu����Ͳ���Fe(x��n)CunNy��FexNyת��Ϊ����Cu����Ͳ���������仯��ͼ2��ʾ�����и��ȶ���Cu����Ͳ���Ļ�ѧʽΪ____��
1-�������һ����Ҫ���л��ϳ��м��壬�е�Ϊ71�棬�ܶ�Ϊ1.36g��cm��3��ʵ�����Ʊ�����1��������Ҫ�������£�����1��������A�м��������ӡ�12g��������20 mLˮ����ˮ��ȴ�»�������28mLŨH2SO4����ȴ�����£������¼���24gNaBr��
����2����ͼ��ʾ�ʵ��װ�ã��������ȣ�ֱ������״�����Ϊֹ��
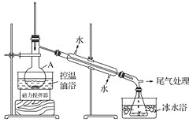
����3�������Һת���Һ©�����ֳ��л��ࡣ
����4�����ֳ����л���ת���Һ©����������12mLH2O��12mL 5% Na2CO3��Һ��12 mL H2Oϴ�ӣ���Һ���ôֲ�Ʒ����һ���ᴿ��1����顣
��1������A��������___�����������ӵ�Ŀ���ǽ����___��
��2����Ӧʱ���ɵ���Ҫ�л���������2������____��
��3������2���������ƿ�ڼ���������ˮ�����ڱ�ˮԡ�е�Ŀ����_____��
��4������2���軺������ʹ��Ӧ������ƽ�Ƚ��У�Ŀ����___��
��5������4����5%Na2CO3��Һϴ���л���IJ��������Һ©����С�ļ���12 mL5%Na2CO3��Һ����___�����ã���Һ��
���𰸡�[Ar]3d5��1s22s22p63s23p63d5 sp2��sp3 9NA H<C<O �Ҵ����Ӽ������� Fe3CuN ������ƿ ��ֹ���� ��ϩ�������� ����1�����Ļӷ� ����HBr�ӷ� ����Һ©���¿�������б�������ų�����
��������
��1������ԭ������Ϊ26��λ�ڵ����������壬![]() ���������Ϊ23�����̬��������Ų�ʽΪ
���������Ϊ23�����̬��������Ų�ʽΪ![]() ��
��![]() ��
��
��2����ͪ�ļ���̼ԭ�ӵ��ӻ���ʽΪ![]() �ӻ����ʻ���̼ԭ�ӵ��ӻ���ʽΪ
�ӻ����ʻ���̼ԭ�ӵ��ӻ���ʽΪ![]() �ӻ���1mol��ͪ�к���6molC-H����2molC-C����1molC=O����������Ϊ�Ҽ���̼��˫���к���1mol�Ҽ�������1mol��ͪ�к���9mol�Ҽ���
�ӻ���1mol��ͪ�к���6molC-H����2molC-C����1molC=O����������Ϊ�Ҽ���̼��˫���к���1mol�Ҽ�������1mol��ͪ�к���9mol�Ҽ���
��3���縺����ԭ���ڷ����������ɼ����ӵ�������ͬ����Ԫ�صĵ縺����ԭ��������������������Ե縺��![]() ������Ϊ�ڼ�����̼Ԫ���Ը����ϼۣ���������������ǿ�����Ե縺��
������Ϊ�ڼ�����̼Ԫ���Ը����ϼۣ���������������ǿ�����Ե縺��![]() ���ʵ縺��
���ʵ縺��![]() ��
��
��4����ͪ���Ӽ�ֻ�з��»��������Ҵ����ڷ��Ӽ������ʹ��е����ߡ�
��5���ɾ���ʾ��ͼ��֪��һ��![]() �����У�������ԭ�ӵ���ĿΪ
�����У�������ԭ�ӵ���ĿΪ![]() ����ԭ�ӵ���ĿΪ1������x=4��y=1����ͼ-2��֪��Cu����þ�����aλ��Fe����ʹ�������ͣ�Cu����þ�����bλ��Fe����ʹ�������ߣ����ȶ���Cu����Ͳ���ΪCu��ȫ����þ�����aλ��Fe����������ͭԭ����ĿΪ
����ԭ�ӵ���ĿΪ1������x=4��y=1����ͼ-2��֪��Cu����þ�����aλ��Fe����ʹ�������ͣ�Cu����þ�����bλ��Fe����ʹ�������ߣ����ȶ���Cu����Ͳ���ΪCu��ȫ����þ�����aλ��Fe����������ͭԭ����ĿΪ![]() ��������ԭ����ĿΪ3���������ȶ���Cu����Ͳ��ﻯѧʽΪ
��������ԭ����ĿΪ3���������ȶ���Cu����Ͳ��ﻯѧʽΪ![]() ��
��
��1������A��������ƿ����������ڴ�������������������ת�ﵽ�����Ŀ�ģ�Ҳ�ܹ�����������г䵱��ʯ�����ã���ֹҺ�屩�С�
��2�����ȹ����У��������ᷢ����������ˮ���ɱ�ϩ����Ӽ���ˮ���������ѡ�
��3������2�е�������¶Ƚϸߣ�����ʹ�ñ�ˮԡ���£����ɵ�1-������ӷ���ʹ�ò��ʽ��͡�
��4������1������е���Ҫ����Ϊ��������HBr�������ȹ�������ʹ�ò���HBr�ӷ����������ȣ��ܹ�������һ���⡣
��5���ֳ����л����лẬ��δ��Ӧ�������ӣ�����![]() ��ˮϴ�Ӻ�����
��ˮϴ�Ӻ�����![]() ���壬��Ҫ����Һ©���¿�������б�������ų����壬Ȼ���÷�Һ��
���壬��Ҫ����Һ©���¿�������б�������ų����壬Ȼ���÷�Һ��



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���߾���G�����������������ϣ��ϳ�G���й�ת����ϵ����
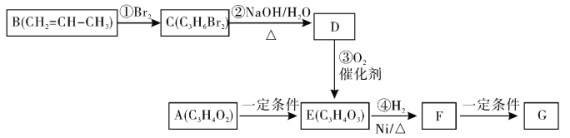
��֪������A�ܹ�����������Ӧ����ش��������⣺
(1)д������D�Ľṹ��ʽ___________��C������Ϊ___________��
(2)F�Ĺ���������Ϊ___________��A��E�ķ�Ӧ����Ϊ��___________��
(3)����˵����ȷ����___________(����ĸ���)��
A.1molE��������H2��һ�������·�����Ӧ���������2molH2
B. 1mol F������NaOH��Һ��Ӧ���������1 molNaOH
C.����B����˳���칹��
D.D��ʹ���Ը��������Һ��ɫ
(4)д������A����������Ӧ�Ļ�ѧ����ʽ��_________________________________��
(5)д��F��G�Ļ�ѧ����ʽ��_________________________________��
(6)������F�ж���ͬ���칹�壬д��������������F�Ķ���ͬ���칹��Ľṹ��ʽ��____��_____��
������NaOH��Һ��Ӧ�����ܷ���������Ӧ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����ͼʵ��װ��������֤ijЩ���ʵ����ʡ����Թ�A��װ�������Ĺ���NaHCO3��DΪ�̶������ӲֽƬ���Իش��������⣺
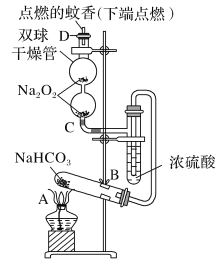
��1����A�Թ��ڷ�����Ӧ�Ļ�ѧ����ʽ��_____��
��2��Bװ�õ�������_____��
��3����˫�������ڷ�����Ӧ�Ļ�ѧ����ʽΪ____��
��4��˫�������ڼ�D���۲쵽��ʵ��������_____��
��5������������ڵ�Na2O2����Na2O����˫�������ڼ�D���۲쵽��ʵ�������ǣ�____��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ������������ȷ����
A. 24 g þ��27 g���У�������ͬ��������
B. ͬ�������������ͳ����У���������ͬ
C. 1 mol��ˮ��1 molˮ�У���������Ϊ2��1
D. 1 mol�����1 mol��ϩ�У���ѧ������ͬ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����һ���¶��£���һ��3L������ܱ�������(Ԥ��װ�����)ͨ��1 mol N2��3 mol H2������һ��ʱ����������ѹǿ����ʼʱ��0.9�����ڴ�ʱ�����v(H2)Ϊ0.1 mol��L��1��min��1������ʱ��Ϊ( )
A. 2 min B. 3 min C. 4 min D. 6 min
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���������ӷ���ʽ��д��ȷ����
A.̼������Һ����μ�������ʵ��������� CO32-+2CH3COOH=CO2��+H2O+2CH3COO-
B.��������ͨ��NaClO��Һ�У�SO2+H2O+2ClO-=SO32-+2HClO
C.��̼�����缫����Ȼ��Ʊ�����Һ 2Cl-+2H2O![]() 2OH-+H2��+Cl2��
2OH-+H2��+Cl2��
D.̼�ᱵ��������� BaCO3+2H+=Ba2++H2O+CO2��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�����ݻ�Ϊ1L���ܱ������У�ͨ��һ������N2O4��������ӦN2O4(g)![]() 2NO2(g)�����¶����ߣ�����������ɫ����ش��������⣺
2NO2(g)�����¶����ߣ�����������ɫ����ش��������⣺
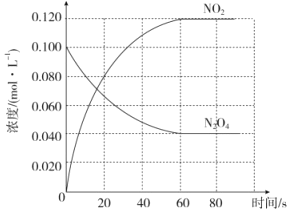
(1)��Ӧ�ġ�H______0(������������С����)��100��ʱ����ϵ�и�����Ũ����ʱ��仯��ͼ��ʾ����0~60sʱ�Σ���Ӧ����v(N2O4)Ϊ________molL-1s-1
(2)100��ʱ�ﵽƽ��ı䷴Ӧ�¶�ΪT��c(N2O4)��0.0020molL-1s-1��ƽ�����ʽ��ͣ�10s�ִﵽƽ�⡣T_______100��(������������С����)��
(3)�¶�Tʱ��Ӧ��ƽ�����Ӧ�������ݻ�����һ�룬ƽ����_______(��������Ӧ�������淴Ӧ��)�����ƶ�
(4)�ٴε���ƽ����������м�����ʵ�����������v��_______v��_______��(����������������С������������)����ϵ����ɫ_______(���������� ����dz������������)��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����֪A�IJ���ͨ����������һ�����ҵ�ʯ�ͻ���ˮƽ������AΪ��Ҫԭ�Ϻϳ�һ�־��й���ζ������E����ϳ�·������ͼ��ʾ��
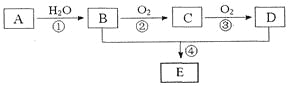
��ش��������⣺
��1��B��D�����еĹ��������Ʒֱ���__________��__________��
��2��д�����з�Ӧ�Ļ�ѧ����ʽ����ע����Ӧ���ͣ�
��____________________��___________����____________________��___________��
��3����ʵ��������B��D�Ʊ�E��ʵ���У�����1mol B��1 mol D��ַ�Ӧ���ܷ�����1mol E_________��ԭ����__________��
��4����184g B��120g D��Ӧ������106g E����÷�Ӧ�IJ���______________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����ͼ��ij��ȤС���̽����ʵ��ͼʾ���й�˵���������ǣ� ��
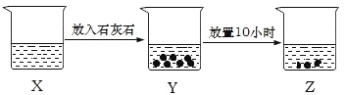
A. ��X����ҺΪFeCl2��Һ����Z�����տ�����Fe(OH)2����
B. ��X����ҺΪ���Ƶı�����ˮ�����ձ���Һ���Ư���ԣ�X��Y��Z
C. ��X����ҺΪNH4Cl��Һ�����ձ���Һ���pH��X��Z
D. ��X����ҺΪAlCl3��Һ����ͨ�����������֤�� Z ���Ƿ����Al(OH)3����
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com