��ˮ���̲��ŷḻ����Դ����ˮ�ۺ����õ�����ͼ���£�
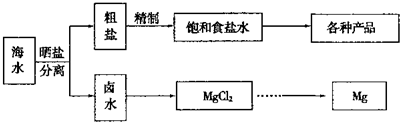
��NaCl��ԭ�Ͽ��Եõ����ֲ�Ʒ��
�ٹ�ҵ����NaCl�Ʊ������ƵĻ�ѧ����ʽ��
��
��ʵ�����ö��Ե缫���500mL0.1mol/LNaCl��Һ���������������õ�112mL���壨��״��������������Һ��pHΪ
12
12
�����Է�Ӧǰ����Һ������仯��
�۵��NaClϡ��Һ���Ʊ���84����Һ����ͨ��ʱ��������������Һ��ȫ���գ�����������Һ����һ�����ʣ�д���õ����ܻ�ѧ����ʽ��
��
�����������ȼ�����Ҫ�ɷ�֮һ�����������Ƶõ������к���������Al
2O
3��SiO
2������ͼ��ʾ��װ�ã��ס��ҹ�����Ƥ�����ӿ������ƶ����ⶨ�����н������ĺ������䷽���ǣ���ϡ���������������Һ����Ʒ���ã�ͨ���������������������Ʒ�н������ĺ���������ʵ��ԭ���ش��������⣺
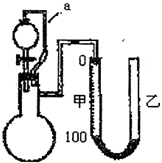
��1��װ���е���a�����������
ƽ����ѹ��ʹ��Һ©���е�Һ��˳������
ƽ����ѹ��ʹ��Һ©���е�Һ��˳������
��
��2��Ϊ�˽�ȷ��������������������ڷ�Ӧǰ���ȡ��Һ��Ķ���ʱӦע��
AD
AD
��
A����Ӧǰ���ȡ��Һ������ʱӦʹ�ס�����Һ�汣��ˮƽ
B����Ӧ���ȡ��Һ������ʱ���ס�������Һ�����뱣��ˮƽ
C����Ӧ��������ȡ��Һ�����ݣ���ֹҺ��䶯
D����Ӧ����һ�ᣬ�����Ӧ���ָ�ԭ���¶�
��3������ϡ���������������Һ����ѡһ���Լ�����ѡ����Լ���
NaOH��Һ
NaOH��Һ
����ѡ����һ���Լ���������
������Ʒ�к���SiO2���������ᷴӦ���в��������ܻ������SiO2�У�����ȷ�����Ƿ�Ӧ��ȫ��ʹʵ��������һ�����
������Ʒ�к���SiO2���������ᷴӦ���в��������ܻ������SiO2�У�����ȷ�����Ƿ�Ӧ��ȫ��ʹʵ��������һ�����
��


 ����ͼ��ʾ��װ�ý��е�⣮ͨ��һ���������ʪ��ĵ���KI��ֽ��C�˱�Ϊ��ɫ��
����ͼ��ʾ��װ�ý��е�⣮ͨ��һ���������ʪ��ĵ���KI��ֽ��C�˱�Ϊ��ɫ��


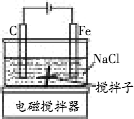
 ij����С��������ͼ��ʾ��װ�ý���ʵ�飮���мס��ҡ�����λͬѧ�ֱ�ѡ�������µ缫���Ϻ͵������Һ��
ij����С��������ͼ��ʾ��װ�ý���ʵ�飮���мס��ҡ�����λͬѧ�ֱ�ѡ�������µ缫���Ϻ͵������Һ�� ����ͼ��ʾ��װ�ý��е�⣮A��ʢ��AgNO3��Һ��B��ʢ�б���Na2SO4��Һͨ��һ���������ʪ��ĵ���KI��ֽ��C�˱�Ϊ��ɫ����
����ͼ��ʾ��װ�ý��е�⣮A��ʢ��AgNO3��Һ��B��ʢ�б���Na2SO4��Һͨ��һ���������ʪ��ĵ���KI��ֽ��C�˱�Ϊ��ɫ����