
����Ŀ�����и�����������Ӧ�������¿��ܴ����������
A. ��ʹpH��ֽ������Һ�У�CO32-��K����Cl����Na��
B. ��ˮ���������c(OH��)��1��10��10 mol��L��1����Һ�У�NO3-��Mg2����Na����SO42-
C. ��c(OH��)/c(H��)��1��1012����Һ�У�NH4+��Fe2����Cl����NO3-
D. Kw/c(H+)��10��10 mol��L��1����Һ�У�Na����HCO3-��Cl����K��
���𰸡�B
��������
����A����ʹpH��ֽ������Һ��������Һ��CO32-��H�������棬A����B����ˮ���������c(OH��)��1��10��10mol/L����Һ����������ҺҲ�����Ǽ���Һ������Һ�и����ӹ��棬����Һ��Mg2����OH�������棬B��ȷ��C�� c(OH��)/c(H��)��1��1012����Һ�Ǽ�����Һ��![]() ��Fe2����OH�������棬C����D��D��
��Fe2����OH�������棬C����D��D��![]() ��10��10mol��L��1����Һ��������Һ��
��10��10mol��L��1����Һ��������Һ��![]() �����ڣ�D����ѡB��
�����ڣ�D����ѡB��


 ����ѧ��Ӯ�����ϵ�д�
����ѧ��Ӯ�����ϵ�д� ѧ���쳵�����ּ��������ҵ�½����������ϵ�д�
ѧ���쳵�����ּ��������ҵ�½����������ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���������������������Ľ��ݣ��ڢ�A����A��VA��Ԫ�������������ϵ���ҪԪ�ء���ش��������⣺
(1)��̬Geԭ�ӵļ۲�����Ų�ͼΪ___________����̬Asԭ�Ӻ�����������ܼ��ĵ�������״Ϊ___________��
(2)Si��P��S��Cl�ĵ�һ�������ɴ�С��˳��Ϊ___________��
(3M��Gaλ��ͬ���ڣ�M3+��һ����������Ϊ[M(NH3)5(H2O)]Cl3��
�������й�NH3��H2O��˵����ȷ����___________(����ĸ)��
a���ӿռ乹����ͬ b����ԭ���ӻ�������ͬ c.���Ǵ�С��ͬ
��1mol[M(NH3)5(H2O)]3+��___________mol������
�������T��[M(NH3)5(H2O)]Cl3���Ԫ����ͬ���������ӵ���λ����ͬ��1molT����ˮ����������AgNO3��Һ������2 mol AgCl����T�Ļ�ѧʽΪ______________________��
(4)̼�������һ�־��壬���۵�Ϊ2870�棬Ӳ�Ƚӽ����ʯ���侧���ṹ��ͼ����ʾ�����仯ѧʽΪ______________________��
(5)����(BP)��һ�ֳ�Ӳ��ĥͿ����ϣ��侧���ṹ��ͼ����ʾ���þ�����Bԭ�������ռ�����Ϊ___________(��������������������������������������)���ռ�������Ϊ___________��
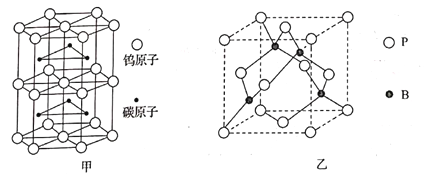
��֪�þ�����ܶ�Ϊ��g��cm��3��NA�ǰ����ӵ�������ֵ��BP������������6��Pԭ�����������������壬����������ı߳�Ϊ___________pm(��ʽ����)��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ʵ��������Ҫ������NaCl��Һ�����ֱ�ֻ�л���Na2SO4��NaHCO3��NaCl��ijѧ���������ͼ��ʾ������ȡ������NaCl��Һ��

����˷�����ȷ����ô��
��1��������Ϊ________�������١��ܡ��ݶ��õ��IJ���������____________��
��2��������Ϊʲô�������ᱵ��Һ����������______________��
��3�����в����ں�����ж�SO42-�ѳ�����������____________________��
��4�������۵�Ŀ����_____________________________________��
��5�������ݵ�Ŀ����_____________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�����и���������ԭ����Ŀ�����ɴ�С˳�����е��ǣ�������
��0.5 mol NH3�ڱ�״����22.4 L He ��4 �� 9 mLˮ ��19.6 g H3PO4
A. �٢ܢۢ� B. �ܢۢڢ�
C. �ڢۢܢ� D. �٢ܢڢ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����1��0.5 mol��Fe��___________����ԭ�ӣ���6.02��1023��ʾ�����ӵ���������������_______g��
��2��2mol CO2�к�______ mol̼ԭ��__________����ԭ��(��NAΪ�����ӵ�������ֵ)��________������(��NAΪ�����ӵ�������ֵ)����״���µ����Ϊ___________L��������______________g��
��3���ڱ�״���£����Ϊ8.96 L��CO��CO2�Ļ�����干14.4 g��
��CO������Ϊ________
�ڻ�������ƽ��Ħ������Ϊ________
��CO2�����Ϊ________
�ܻ��������ܶ�Ϊ_____��С�������1λ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����N2��H2����ʵ��NH3�Ĺ�ҵ�ϳɣ������ֿ��Խ�һ���Ʊ����ᣬ�ڹ�ҵ��һ��ɽ���������������ش��������⣺
��1����֪��N2(g)+O2(g) = 2NO(g)��H=+180��5kJ/mol��N2(g)+3H2(g) ![]() 2NH3(g)��H=��92��4kJ/mol��2H2(g)+O2(g) = 2H2O(g) ��H=��483��6kJ/mol��д����������������ȫ����һ�����������ˮ�������Ȼ�ѧ����ʽΪ_________��
2NH3(g)��H=��92��4kJ/mol��2H2(g)+O2(g) = 2H2O(g) ��H=��483��6kJ/mol��д����������������ȫ����һ�����������ˮ�������Ȼ�ѧ����ʽΪ_________��
��2��N2O5��һ�������������������ʺ��Ʊ��ܵ����ǵĹ�ע������H2��O2��������Na2CO3��ɵ�ȼ�ϵ�أ����õ�ⷨ�Ʊ�N2O5��װ����ͼ��ʾ������YΪCO2��
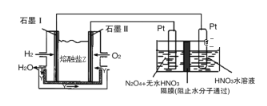
д��ʯīI�缫�Ϸ�����Ӧ�ĵ缫��Ӧʽ_______________________________���ڵ���������N2O5�ĵ缫��ӦʽΪ__________________________________��
(3)�Լ״�ȼ�ϵ��Ϊ��Դ���ö��Ե缫��ⱥ��NaCl��Һʱ��ÿ����0.2mol CH3OH�����������������������Ϊ________L��
��4����һ��������ܱ������У��������»�ѧ��Ӧ�� N2(g)+3H2(g) ![]()
![]() 2NH3(g)���仯ѧƽ�ⳣ��K���¶�t�Ĺ�ϵ���±���
2NH3(g)���仯ѧƽ�ⳣ��K���¶�t�Ĺ�ϵ���±���
t/K | 298 | 398 | 498 | �� |
K/(mol��L��1)2 | 4.1106 | K1 | K2 | �� |
����������⣺
����֪�÷�Ӧ�ܹ��Է����У��ԱȽ�K1��K2�Ĵ�С��K1_______ K2���>������=����<������
����ͬ��ͬѹʱ�жϸ÷�Ӧ�ﵽ��ѧƽ��״̬��������_________������ţ���
A��2v(H2)������=3v(NH3)���棩 B��2v(N2)������=v(H2)���棩
C��������ѹǿ���ֲ��� D�����������ܶȱ��ֲ���
��5�������£�N2H6Cl2����һ����Ҫ�Ļ���ԭ�ϣ��������ӻ����������ˮ����Һ�����ԣ�ˮ��ԭ����NH4Cl���ơ�
��д�������µ�һ��ˮ�ⷴӦ�����ӷ���ʽ__________________________________��
��������ˮ��Һ������Ũ�ȵ�����˳����ȷ����__________������ţ���
A��c(Cl��)>c(N2H62+)> c(OH��)> c(H+)
B��c(Cl��)>c([N2H5��H2O]+)> c(H+)>c(OH��)
C��2 c(N2H62+)+ c([N2H5��H2O]+)+c(H+)= c(Cl��)+c(OH��)
D��c(N2H62+)> c(Cl��)>c(H+)>c(OH��)
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����98%��Ũ���ᣨ���ܶ�Ϊ1.84g/cm3������100mL 1.0mol��L1ϡ���ᣬ��ʵ�������У�
A��100mL��Ͳ B��������ƽ C�������� D��50mL����ƿ E��10mL��Ͳ F��50mL�ձ� G��100mL����ƿ
��1����ɸ�ʵ�黹ȱ�ٵ�������______��
��2����ʵ������ȡŨ��������Ϊ___mL��
��3��������ƿʹ�÷����У����в�������ȷ����_____������ţ���
A��ʹ������ƿǰ������Ƿ�©ˮ
B�����������ƹ��������ƽ���̵���ֽ�ϣ�ȷ�����������ձ����ܽ������ע������ƿ��
C����ȷ��ȡ��18.4mol��L1�����ᣬע����ʢ��30mLˮ��100mL������ƿ�У���ˮ���̶���
D�����ݺ�����ƿ������ʳָ��סƿ��������һֻ�ֵ���ָ��סƿ�ף�������ƿ��תҡ��
��4���������ʹ�����Ƶ�ϡ����Ũ��ƫ����_____ ������ţ���
A�����õ�Ũ���᳤ʱ��������ܷⲻ�õ�������
B������ƿ������ˮϴ�Ӻ������������ˮ
C������ʱ������Һ�İ�Һ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����֪A��B��C��D����ѧ��ѧ�������ʣ�������һ����������A+B��C+D��ת����ϵ��
��1����AΪ��������BΪ���������÷�Ӧ��һ����;��_______________��
��2����A��һ����ʹʪ��ĺ�ɫʯ����ֽ���������壬�Ҹ÷�Ӧ�ǹ�ҵ����ȡ�������Ҫ��Ӧ֮һ���÷�Ӧ�Ļ�ѧ��Ӧ����ʽΪ____________________________��
��3����A�ǵ���ɫ��ĩ����������������CΪǿ���÷�Ӧ�Ļ�ѧ��Ӧ����ʽΪ_______��
��4����A��B��D�����л����������A��B�Ǽ�ͥ�����г�����ζƷ����Ҫ�ɷ֣���A ����Է���������B��14��
�ٸ÷�Ӧ�Ļ�ѧ��Ӧ����ʽΪ_____________________��
��ij����BΪ��Ӧ������͵����ͼ��ʾ���õ�صĸ����ĵ缫��ӦʽΪ_________��

��5��ClO2�ɽ������Է�ˮ�е�Mn2+ת��ΪMnO2����ȥ��ͬʱClO2����ԭΪCl�����÷�Ӧ�����ӷ���ʽΪ________________________________��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com