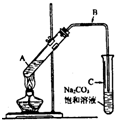
��2009?�ɶ�һģ��ij��ȤС��������ͼװ�ã�ȡ��ͬŨ�ȵ������� 3mL ��ˮ�Ҵ��� 2mL ������ֱ������ȡ�����������о���
��1��A �Թ����������������Ļ�ѧ��Ӧ����ʽΪ��
CH
3COOH+HOCH
2CH
3
CH
3COOCH
2CH
3+H
2O
CH
3COOH+HOCH
2CH
3
CH
3COOCH
2CH
3+H
2O
��
��2�������� B �Ĺܿ�û�в��뱥��̼����Һ�����£�ԭ����
��ֹ̼����Һ�嵹��������������������
��ֹ̼����Һ�嵹��������������������
��
��3����ȤС���¼��ʵ������ͽ�������
| ����� |
���� |
��Ӧ���� |
C�б���̼������Һ������߶� |
| �� |
2mL98%Ũ���� |
20��ʱ��Һ������ɫ���淴Ӧ���У���Һ��ɫ������ɺ�ɫ�����������ݣ� |
2.10cm |
| �� |
2mL14mol?L-1���� |
��Ӧ����Һ��ɫ����ɫ�������뱥��̼������Һ���������������ݣ� |
2.14cm |
| �� |
2mL10mol?L-1���� |
��Ӧ����Һ��ɫ����ɫ�������뱥��̼������Һ���������������ݣ� |
2.16cm |
| �� |
2mL7mol?L-1���� |
��Ӧ����Һ��ɫ����ɫ�������뱥��̼������Һ�����ݣ� |
2.00cm |
�� ����ʵ���У��Թ�����Һ��ɫ�淴Ӧ�����������ɺ�ɫ��ԭ���ǣ�
98%��Ũ�������ǿ�����ԣ�ʹ���ַ�Ӧ��̿��
98%��Ũ�������ǿ�����ԣ�ʹ���ַ�Ӧ��̿��
����ͬʱ�д̼�����ζ���������������ܷ����Ļ�ѧ��Ӧ����ʽ�ǣ�
CH
3CH
2OH+2H
2SO
4��Ũ��
2C��+2SO
2��+5H
2��
CH
3CH
2OH+2H
2SO
4��Ũ��
2C��+2SO
2��+5H
2��
��
���Թ� C ��������û�������ʵ�����ǣ��������
�٢ڢ�
�٢ڢ�
����ʵ����������ѡ�ô��������Ũ����
10 mol?L-1
10 mol?L-1
��
��4���C
4H
8O
2�����������ͬ���칹��Ľṹ��ʽ
HCOOCH2CH2CH3��HCOOCH��CH3��2��CH3COOCH2CH3��CH3CH2COOCH3
HCOOCH2CH2CH3��HCOOCH��CH3��2��CH3COOCH2CH3��CH3CH2COOCH3
��






 +5H2
+5H2
 +5H2
+5H2


 +n
+n

 +n
+n

 ��2009?�ɶ�һģ��ij��ȤС��������ͼװ�ã�ȡ��ͬŨ�ȵ������� 3mL ��ˮ�Ҵ��� 2mL ������ֱ������ȡ�����������о���
��2009?�ɶ�һģ��ij��ȤС��������ͼװ�ã�ȡ��ͬŨ�ȵ������� 3mL ��ˮ�Ҵ��� 2mL ������ֱ������ȡ�����������о��� CH3COOCH2CH3+H2O
CH3COOCH2CH3+H2O CH3COOCH2CH3+H2O
CH3COOCH2CH3+H2O