
����Ŀ����1��һ�������£������Ժ�![]() ������Ӧ��
������Ӧ��![]() ����֪�÷�Ӧ��ƽ�ⳣ��
����֪�÷�Ӧ��ƽ�ⳣ��![]() ���¶�
���¶�![]() �Ĺ�ϵ��ͼ��ʾ��
�Ĺ�ϵ��ͼ��ʾ��
�ٸ÷�Ӧ��_______________�������������������������ķ�Ӧ��
���ں��º��ݵ������н��и÷�Ӧ��������˵����Ӧ�ﵽƽ��״̬����_____________������ĸ���ţ�
A. ![]() ��Ũ�ȱ��ֲ���
��Ũ�ȱ��ֲ���
B.��������ѹǿ���ٱ仯
C. ![]()
D.��������ƽ����Է����������ٱ仯
��![]() �¶��£������Ϊ
�¶��£������Ϊ![]() ���ܱ������м����������۲�����һ������
���ܱ������м����������۲�����һ������![]() ����
����![]() ��ƽ��ת����Ϊ_______��
��ƽ��ת����Ϊ_______��
��2���״�ȼ�ϵ���У����������Һ��![]() �����ʵ���Ϊ
�����ʵ���Ϊ![]() ������
������![]() �״����뷴Ӧʱ��������Һ������Ũ���ɴ�С��˳����_________________________��
�״����뷴Ӧʱ��������Һ������Ũ���ɴ�С��˳����_________________________��
��3����![]() ��
��![]() �ɷ��Ʊ�
�ɷ��Ʊ�![]() �ķ�Ӧ�����а������Ȼ�ѧ����ʽ�У�
�ķ�Ӧ�����а������Ȼ�ѧ����ʽ�У�
![]()
![]()
![]()
![]()
��Ӧ![]() ��
��![]() ______
______![]() ���ú�
���ú�![]() �Ĵ���ʽ��ʾ����
�Ĵ���ʽ��ʾ����![]() ����1841�����ô������缫����Ũ��
����1841�����ô������缫����Ũ��![]() ��Һ����Ƶ�
��Һ����Ƶ�![]() ����������
����������![]() �ĵ缫��ӦʽΪ___________��
�ĵ缫��ӦʽΪ___________��![]() �ȶ�����иĽ������������缫�����������ӽ���Ĥ����Ч����˲��ʣ������ӽ���Ĥ��������________��
�ȶ�����иĽ������������缫�����������ӽ���Ĥ����Ч����˲��ʣ������ӽ���Ĥ��������________��
���𰸡����� ![]() 75%
75% ![]()
![]()
![]() ==
== ![]() ����
����![]() �������ϱ���ԭ
�������ϱ���ԭ
��������
��ѧƽ�ⳣ��ֻ���¶��йأ������ǵĹ�ϵ���жϷ�Ӧ����ЧӦ����ѧƽ��ı��������淴Ӧ������ȣ������Ǹ����ʵ�Ũ�ȵȱ��ֲ��䣬�ɴ��ж��Ƿ�ﵽ��ѧƽ��״̬����ƽ�ⳣ���ɼ���ƽ��ת���ʡ���֪�״����������ص����ɼ��㷴Ӧ�����ʵ����ʵ������پ�ˮ��̶ȵ����ι�ϵ���Ƚ�����Ũ�ȴ�С���ݸ�˹���ɣ�����֪�Ȼ�ѧ����ʽ�Ӽ������������Ŀ���Ȼ�ѧ����ʽ�����ݵ��ԭ����д�缫��Ӧʽ�����������ӽ���Ĥ�����á�
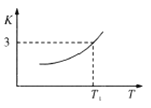
��1���ٸ���ͼ���¶����ߣ�ƽ�ⳣ���������Ը÷�Ӧ�����ȷ�Ӧ��
�ھݷ�Ӧ![]() ��
��![]() .�÷�Ӧ���ҽ��У�����������
.�÷�Ӧ���ҽ��У�����������![]() ��Ũ�ȱ��ֲ��䣬˵���ﵽƽ��״̬��
��Ũ�ȱ��ֲ��䣬˵���ﵽƽ��״̬��![]() ��ȷ��
��ȷ��![]() .�÷�Ӧ����������������仯�ķ�Ӧ��������ѹǿʼ�ձ��ֲ��䣬
.�÷�Ӧ����������������仯�ķ�Ӧ��������ѹǿʼ�ձ��ֲ��䣬![]() ����
����![]() .
.![]() �ǹ��壬����ʾ��ѧ��Ӧ���ʣ�
�ǹ��壬����ʾ��ѧ��Ӧ���ʣ�![]() ����
����![]() .�÷�Ӧ���ҽ��У�ƽ����Է����������٣���������ƽ����Է����������ٱ仯ʱ����Ӧ�ﵽƽ��״̬��
.�÷�Ӧ���ҽ��У�ƽ����Է����������٣���������ƽ����Է����������ٱ仯ʱ����Ӧ�ﵽƽ��״̬��![]() ��ȷ��ѡ��AD��
��ȷ��ѡ��AD��
�۸��ݼ�ͼ��֪��![]() �¶���
�¶���![]() �������
�������![]() �����ʵ���Ϊ
�����ʵ���Ϊ![]() ���跴Ӧ��
���跴Ӧ��![]()
![]()
![]() ��������
��������![]() ������
������![]() ��
��![]() �����
�����![]() ��������
��������![]() ��ƽ��ת����Ϊ
��ƽ��ת����Ϊ![]() ��
��
��2������![]() �״����뷴Ӧ�������Ķ�����̼��
�״����뷴Ӧ�������Ķ�����̼��![]() ����
����![]() �������ط�Ӧ������Ԫ���غ���Լ���
�������ط�Ӧ������Ԫ���غ���Լ���![]() �����ߵ�ˮ��Һ��Ϊ���ԣ�������Һ������Ũ�ȴ�С˳��Ϊ
�����ߵ�ˮ��Һ��Ϊ���ԣ�������Һ������Ũ�ȴ�С˳��Ϊ![]() ��
��
��3������֪�Ȼ�ѧ����ʽ���϶��±��Ϊ�٢ڢۢ�������Ŀ�귽��ʽ����ȥ�����ʡ������й����ʣ���Ŀ�귽��ʽ����+��/2+��/2+������![]() ��
��
�������缫���Ũ![]() ��Һ�Ƶ�
��Һ�Ƶ�![]() ����Ԫ�ش�0������+6�ۣ�������ΪFeʧ��������
����Ԫ�ش�0������+6�ۣ�������ΪFeʧ��������![]() ���缫��ӦʽΪ
���缫��ӦʽΪ![]() ��
��![]() �������ӽ���Ĥ������������ͨ�����������ɵ�
�������ӽ���Ĥ������������ͨ�����������ɵ�![]() ��������������ԭ���Ӷ����
��������������ԭ���Ӷ����![]() ���ʡ�
���ʡ�


 ���ݼ���ϵ�д�
���ݼ���ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ͨ����ˮ��ɹ�ɵô��Σ����γ�NaCl�⣬������MgCl2��CaCl2��Na2SO4�Լ���ɳ�����ʡ��������Ʊ����ε�ʵ�鷽������������������ͼ��

��1���ڵڢٲ������ܽ������Ҫ�ò��������裬������______��
��2���ڢڲ�������Ŀ���dz�ȥ�����е�______���ѧʽ����ͬ�����ڢ�������Ŀ���dz�ȥ��Һ��______��
��3���ڢݲ������ˡ������еõ������ijɷ��У���ɳ��BaSO4��Mg��OH��2��______���ѧʽ����
��4���ڵڢ۲������У�ѡ��ij��ӵ��Լ�������KOH����NaOH��������______��
��5��ʵ�����þ��ε��������ڴ�����NaCl��������ԭ����______��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��M(NO3)2�ȷֽ⻯ѧ����ʽΪ2M(NO3)2![]() 2MO+4NO2��+ O2��������29.6 g M(NO3)2ʹ����ȫ�ֽ⣬�ڱ�״�����ռ�11.2 L�����壬��ôM��Ħ��������
2MO+4NO2��+ O2��������29.6 g M(NO3)2ʹ����ȫ�ֽ⣬�ڱ�״�����ռ�11.2 L�����壬��ôM��Ħ��������
A.24 g��mol-1B.74 g��mol-1
C.148 g��mol-1D.40 g��mol-1
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�������������ɵ����������ϳɰ����ķ��������1918��ŵ������ѧ��������һ�ܱ������г���1mol N2��3mol H2����һ��������ʹ�÷�Ӧ������N2��3H2![]() 2NH3�������й�˵����ȷ���ǣ� ��
2NH3�������й�˵����ȷ���ǣ� ��
A. �ﵽ��ѧƽ��ʱ������Ӧ���淴Ӧ�����ʶ�Ϊ��
B. �����ϣ�3��N2����H2ʱ����Ӧ�ﵽƽ��״̬
C. �ﵽ��ѧƽ��ʱ����λʱ������a molN2��ͬʱ����3a molH2
D. ��N2��H2��NH3�ķ�������Ϊ1��3��2����Ӧ�ﵽƽ��״̬
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�����и�������ȷ������ ��
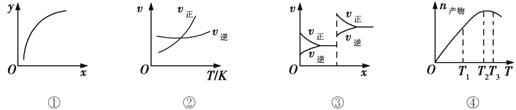
A. ͼ����ʾ�ں���������MgSO4(s) + CO(g)![]() MgO(s) + CO2(g) + SO2(g)���������������þ��������������ɴ���CO��ת����
MgO(s) + CO2(g) + SO2(g)���������������þ��������������ɴ���CO��ת����
B. ͼ����ʾ��������һ��ʱ����ӦA(g)+3B(g)![]() 2C(g)�ķ�Ӧ�������¶ȱ仯��ͼ������Ӧ��H>0
2C(g)�ķ�Ӧ�������¶ȱ仯��ͼ������Ӧ��H>0
C. ͼ����Ӧ�ķ�Ӧһ���Ƿǵ������Ӧ��ѹ��ƽ���ƶ��ı仯���
D. ��ѹ�ܱ������м���һ����A��B��������ӦA(g)+3B(g)![]() 2C(g)��ͼ����ʾ��Ӧ�����в��������¶ȣ�����C���ʵ����仯���ɣ�������Ӧ��������
2C(g)��ͼ����ʾ��Ӧ�����в��������¶ȣ�����C���ʵ����仯���ɣ�������Ӧ��������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���ش��������⣺
��1����֪������CO��ȼ����Ϊ283 kJ/mo1����CO��ȼ���ȵ��Ȼ�ѧ����ʽΪ___________________________________��
��2����ҵ������CO��H2�ϳ������ԴCH3OH���䷴ӦΪ��CO(g)+2H2(g)![]() CH3OH(g) ��H=-116kJ/mo1
CH3OH(g) ��H=-116kJ/mo1
��ͼ��ʾCO��ƽ��ת����(��)���¶Ⱥ�ѹǿ�仯��ʾ��ͼ��X��ʾ����_____________��Y1_____Y2(�<������=������>��)��
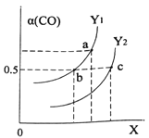
��3���ϳɼ״��ķ�Ӧԭ��Ϊ��CO2(g)+3H2(g)![]() CH3OH(g)+H2O(g)����1L���ܱ������У�����1mol CO2��3mol H2����500���·�����Ӧ�����CO2(g)��CH3OH(g)��Ũ����ʱ��仯��ͼ��ʾ��
CH3OH(g)+H2O(g)����1L���ܱ������У�����1mol CO2��3mol H2����500���·�����Ӧ�����CO2(g)��CH3OH(g)��Ũ����ʱ��仯��ͼ��ʾ��

�ٷ�Ӧ���е�4minʱ��v(��)____v(��)(�>����<����=��)��0~4min��CO2��ƽ����Ӧ����v(CO2)=____________mol��L1��min1��
�ڸ��¶���ƽ�ⳣ��Ϊ_____________��
��������˵���÷�Ӧ�Ѵﵽƽ��״̬����______________��
A��v��(CH3OH)=3v��(H2)
B��CO2��H2��CH3OH��H2OŨ��֮��Ϊ1��3��1��1
C�����º�ѹ�£������������ٱ仯
D�����º����£�������ܶȲ��ٱ仯
��4��Ϊ���ȼ�ϵ����������ʣ����������Ϊȼ�ϵ�ء�ij����Լ���Ϊȼ�ϣ�����Ϊ��������KOH��ҺΪ�������Һ���Ծ��д����ú͵������ܵ�ϡ������Ϊ�缫��д����ȼ�ϵ�صĸ�����Ӧʽ��_____________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�������л���![]()
![]() ��˵����ȷ����
��˵����ȷ����
A. a��b��Ϊͬϵ��
B. c������̼ԭ�ӿ��ܴ���ͬһƽ��
C. a��b��c����ʹ���Ը��������Һ��ɫ
D. b��ͬ���칹���к����Ȼ��Ľṹ����7��(���������칹)
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ij������A���Դ�ú����õ���ú�����з����������AΪԭ�Ͽ��Ժϳɾ��ڰ��������ᡢ����������ʣ���ϳ���������(���ֲ���ϳ�·�ߡ���Ӧ��������ȥ)��
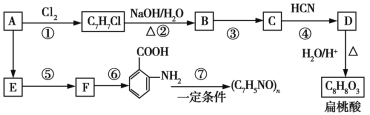
��֪��
��R��CHO+HCN![]()
![]()
��R��CN![]() R��COOH
R��COOH
��![]()
![]()
![]() (�����ױ�����)
(�����ױ�����)
��ش��������⣺
(1)C�ķ���ʽΪ__________��
(2)���ж���ط�Ӧ���͵��жϺ�������__________ (�����)��
�� | �� | �� | �� | �� | �� | �� | |
�� | �ӳ� | ˮ�� | ��ԭ | ȡ�� | ��ԭ | ���� | �Ӿ� |
�� | �ӳ� | ��ȥ | ��ԭ | �ӳ� | ���� | ��ԭ | ���� |
�� | ȡ�� | ˮ�� | �ӳ� | ���� | ��ԭ | ���� | |
�� | ȡ�� | ��ȥ | ���� | ȡ�� | ��ԭ | ���� | �Ӿ� |
(3)д����Ӧ�۵Ļ�ѧ����ʽ��______________________________��
(4)�������ж���ͬ���칹�壬���м������Ȼ�����Һ������ɫ��Ӧ��������̼��������Һ��Ӧ�������ݵ�ͬ���칹����__________�֣�д������һ�ֵĽṹ��ʽ��__________________��
(5)�Է�����AΪ��Ҫԭ�ϣ�������ͨ�����кϳ�·�ߺϳɰ�˾ƥ�ֺͶ����ͣ�

�ٶ����͵Ľṹ��ʽΪ____________________��
��д����Ӧ���Ļ�ѧ����ʽ��______________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����Ȼ���з����Ի���̬��ʽ���ڣ���Ҫ��өʯ(CaF2)������ʯ(Na3AlF6)��
(1)��̬��ԭ���У���_______��������ͬ�ĵ��ӡ�
(2)өʯ(CaF2)������ˮ���������ں�Al3������Һ�У�ԭ����_________________�������ӷ���ʽ��ʾ��������֪AlF63-����Һ�п��ȶ����ڣ�
(3)BF3��һ������ˮ�γ�(H2O)2BF3����Q������Q��һ�������¿�ת��ΪR��

�پ���R�к��еĻ�ѧ������_________�����ţ���
A�����Ӽ� B����λ�� C�����ۼ� D�����
��R�������ӵĿռ乹��Ϊ_________������������ԭ�ӵ��ӻ���ʽΪ_________��
(4)F2������±�ص��ʷ�Ӧ�����γ�±�ػ������ClF3��BrF3�ȡ�ClF3���۷е��BrF3�ĵͣ�ԭ����___________________________________��
(5)Na3AlF6�����ṹ��ͼ��ʾ��

��λ�ڴ����������Ĩ�����________�������ӷ��ţ���
��AlF63-�������������ܶѻ��γ���������������������ֿ�϶�����������϶����AlF63-��֮��Ϊ_______������_________%���������϶��Na+��䣻
�۾����߳�Ϊx nm����������������Na+֮�����Ϊ_____ nm����Na3AlF6����Է�������ΪM�������ӵ�����ΪNA����þ����ܶȵļ������ʽΪ________g/cm3��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com