


 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 ij��ȤС��ͬѧ��ʵ�����ü���l-������ŨH2SO4���廯�ƻ����ķ������Ʊ�1-�嶡�飬�������ͼ��ʾ��ʵ��װ�ã����еļг�����û�л�������
ij��ȤС��ͬѧ��ʵ�����ü���l-������ŨH2SO4���廯�ƻ����ķ������Ʊ�1-�嶡�飬�������ͼ��ʾ��ʵ��װ�ã����еļг�����û�л�������| Ũ���� |
| ���� |
| Ũ���� |
| ���� |
| ���� | �۵�/�� | �е�/�� |
| 1-���� | -89.5 | 117.3 |
| 1-�嶡�� | -112.4 | 101.6 |
| ���� | -95.3 | 142.4 |
| 1-��ϩ | -185.3 | -6.5 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| M |
| NA |
| M |
| NA |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| ʵ���� | HA���ʵ���Ũ�ȣ�mol*L-1�� | NaOH���ʵ���Ũ�ȣ�mol*L-1�� | �����Һ��pH |
| �� | 0.1 | 0.1 | pH=9 |
| �� | c | 0.2 | pH=7 |
| �� | 0.2 | 0.1 | pH��7 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 �۰��� ������
�۰��� ������ ����ˮ ��12C
����ˮ ��12C�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
| ��ѧʽ | HF | H2CO3 | HClO |
| ����ƽ�ⳣ�� ��K�� |
7.2��10-4 | K1=4.4��10-7 K2=4.7��10-11 |
3.0��10-8 |
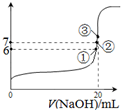 ������˵����ȷ����
������˵����ȷ���� SO2Cl2��l����H=-97.3kJ?mol-1
SO2Cl2��l����H=-97.3kJ?mol-1�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com