
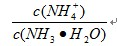 ��ֵ
��ֵ| c(HCO3-)?c(OH-) |
| c(CO32-) |
| c(HCO3-)?c(OH-)?c(H+) |
| c(CO32-)?c(H+) |
| KW |
| K2 |
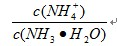 =
=| c(H+) |
| K(�����ˮ��ƽ�ⳣ��) |
| c(HCO3-)?c(OH-) |
| c(CO32-) |
| c(HCO3-)?c(OH-)?c(H+) |
| c(CO32-)?c(H+) |
| KW |
| K2 |
| 10-14 |
| 5.6��10-11 |
| c(NH4+)c(OH-) |
| c(NH3?H2O) |
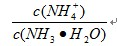 =
=| c(H+) |
| K(�����ˮ��ƽ�ⳣ��) |


 ��У����ϵ�д�
��У����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�����£���Ũ�Ⱦ�Ϊ0.1 mol/L��4����Һ�������������۴��
��������������ע��������Һ���ʱ����ı仯��
��1����������Һ�����������Ϻ���ҺpH=7������У�����ţ� ��
��2�����ᱵ��������ˮ��ǿ����ʡ�������ܵ������ϣ�������Һ������Ũ���ɴ�С��˳���� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2010-2011ѧ�걱����������������ѧ����ĩ���Ի�ѧ�Ծ� ���ͣ������
�����£���Ũ�Ⱦ�Ϊ0.1 mol/L��4����Һ�������������۴��
��������������ע��������Һ���ʱ����ı仯��
��1����������Һ�����������Ϻ���ҺpH=7������У�����ţ� ��
��2�����ᱵ��������ˮ��ǿ����ʡ�������ܵ������ϣ�������Һ������Ũ���ɴ�С��˳���� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�����£���Ũ�Ⱦ�Ϊ1 mol��L��1������4����Һ��
��H2SO4��Һ ��NaHCO3��Һ ��NH4Cl��Һ ��NaOH��Һ
��1����4����ҺpH�ɴ�С��˳���� ��������ˮ�����H��Ũ����С���� ����������ţ�
��2�����и�����Ũ���ɴ�С��˳���� ��NaHCO3��ˮ��ƽ�ⳣ��Kh�� mol��L��1������֪̼��ĵ��볣��K1��4��10��7��K2��5.6��10��11��
��3�������ͨ��������������ʱ![]() ��ֵ �����������С�����䡱����
��ֵ �����������С�����䡱����
��4�������ۺܻ͢�Ϻ���Һǡ�ó����ԣ�����ǰ�۵���� �ܵ����������ڡ�����С�ڡ����ڡ�֮һ����
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com