
��1���ݡ��Ƽ���Ϣ�������������ڷ����ŷ���ʹ�����ϼ��Ͽյ�a�������400����ƽ������Ŀն����к������߳���ֱ�룬��b�ֽ⣬�ܶȽ�С����ͻ����ݵ�����ռ䣬ʣ�µ������ջ�ʹ����c���Ӷ�ʹ�������������ص����ǵĸ��ޡ����������������ش����⡣
����䣺a____b____c_____��
��д��a��b��Ӧ�Ļ�ѧ����ʽ��a_________b________��
��2���ݡ��й�����������������һ�ݿƼ����ؿ����о������ʾ���ҹ���������ռ���������40%���о������������������ҹ�ũ���ɭ�ֵ�Ӱ�������
��ͼ���о���������ѧ��ʵ���õġ����������������������в����ܵĴֵ��ߴ�����Ƥ�����̶��ڴֲ������У�������һ���õ���˿�������������Ƴɡ���������

ʵ��ʱ���Ƚ�ֱͨ����Դ��ʹ����˿���ȣ�Ȼ���ȵĵ���˿����װ��SO2�Ϳ����ļ���ƿ�У�ƿ����������_____ �����������м��������ữ���Ȼ�����Һ���ֳ���______����
��ش�
������ʵ���пɵó��������Ļ�ѧԭ����_________��
��Ŀǰһ���еȳ���ÿ����úԼ300��֣��京�����簴1%���㣬��ÿ���ŷ�SO2���ٶ֣����˶���������60%ת��Ϊ���ᣬ�൱�����ɶ��ٶ�98%���
��Ϊ�˷������꣬����úȼ��ʱ������ŷŵ�SO2����ҵ�Ͻ���ʯ�Һͺ���ú��Ϻ�ʹ�á���д��ȼ��ʱ���йء�������ʹ��Ļ���������������Ӧ�Ļ�ѧ����ʽ______,���Ƚϴ˷��뽫��ʯ��ʯ��δ�뺬��ú��ϡ�ʹ�÷�������ķ������ĸ�����Щ����_________�����ʯ�ҷ�����ʯ��ʯ������
����ÿ��һ��ʱ��ⶨij����ˮ�����꣩��Ʒ��pH���������й�ʱ��������pH�仯������ͼ��
�ݹ��������²��á���������������Һ���շ�������д���йط�Ӧ�Ļ�ѧ����ʽ______��
��1���ٳ����㣻ˮ���ữ����2O3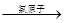 3O2��2H2O
3O2��2H2O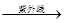 2H2��+O2��
2H2��+O2��
��2���ٿ����е�SO2������ú�̵ȶ�SO2�������д����õĿ����ͻᱻ�����γ����ꡣ
��6��֣�56250t��
��CaO+SO2=CaSO3, 2CaSO3+O2="2" CaSO4����ʯ�ҷ���
��
��Na2SO3+SO2+H2O=2NaHSO3��2NaHSO3= Na2SO3+SO2+H2O��
��������
�����������1���ٸ�����֪�������Ϣ���жϣ�a��b��c�ֱ��dz����㡢ˮ��������
��a��b�ֱ����dz�����ˮ�ķֽ⣬���Է�Ӧ�Ļ�ѧ����ʽ�ֱ���2O3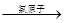 3O2��2H2O
3O2��2H2O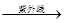 2H2��+O2����
2H2��+O2����
��2�������ڿ����е�SO2������ú�̵ȶ�SO2�������д����ã����Է�Ӧ�����������ɣ��Ӷ��γ����ꡣ
��ÿ���ŷ�SO2�������� ��
��
SO2 �� H2SO4
64t 98t
60000t��60% m��98%
���m��56250t
��SO2������������ܺ���ʯ�ҷ�Ӧ����������ƣ�������Ʋ��ȶ����ױ�����������������ƣ���Ӧ�Ļ�ѧ����ʽ��CaO+SO2=CaSO3, 2CaSO3+O2="2" CaSO4�����ڴ���ʯ�ڷֽ�ʱ������CO2���������ЧӦ��������ʯ�ҷ��Ϻá�
������ʱ������ƣ������е�����������������ǿ�����ᣬ����pH���ͣ����ղ��ٷ����仯������ͼ����
����������������SO2�������������ƣ����������Ʒֽ��ֲ���SO2�����Է�Ӧ�Ļ�ѧ����ʽ�ֱ���Na2SO3+SO2+H2O��2NaHSO3��2NaHSO3�� Na2SO3+SO2+H2O��
���㣺���������γɵ�ԭ�������Ρ��Լ��йؼ���
�������������е��Ѷȵ����⣬���������ǿ���������У����ؿ���ѧ���������⡢����������������������������ѧ���Ļ���������ʶ����ǿѧ����������θС�



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
(1)��������Խ��Խ�������ǹ㷺�Ĺ�ע����ѧʵ���г�������к����壬������ȡijЩ��ʩ����ֹ�ͼ��ٴ�����Ⱦ���磺����������� ���գ�����İ����ý��ݹ� ��Һ������������Ũ�����������ʵ��ʱ�������ܿڲ���һ��պ�� ���������� ���塣
(2)�ݡ��Ƽ���Ϣ�������������ڷ����ŷ���ʹ�����ϼ��Ͽյ�a�������400����ƽ��ǧ�Ŀն����к��������߳���ֱ�룬��b�ֽ⣬�ܶȽ�С����ͻ����ݵ�����ռ䣬ʣ�µ������ջ�ʹ����c���Ӷ�ʹ�������Ҳ��������������������С���ˮ���Ļ�Į�����������������ش����⣺
����գ�a�� ��b�� ;c�� ��
��д��a��b��Ӧ�Ļ�ѧ����ʽ��
a�� �� b�� ��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com