
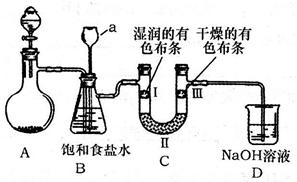
�������ȣ�ClO2����һ�ֻ���ɫ�д̼�����ζ�����壬���۵�Ϊ��59�棬�е�Ϊ11.0�棬������ˮ����ҵ���ó�ʪ��KClO3�Ͳ��ᣨH2C2O4����60ʱ��Ӧ�Ƶá�ijѧ����������ͼ��ʾ��װ��ģ����ȡ���ռ�ClO2��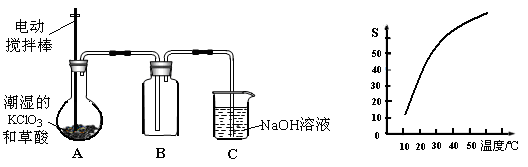
��1��A�з�Ӧ������K2CO3��ClO2��CO2�ȣ���д���÷�Ӧ�Ļ�ѧ����ʽ�� ��
��2��A���������¶ȿ���װ�ã����ƾ����⣬����Ҫ�IJ����������ձ��� ��BҲ���������¶ȿ���װ�ã�Ӧ���� (ѡ���ˮԡ������ˮԡ��)װ�á�
��3����Ӧ����װ��C�пɵ�NaClO2��Һ����֪NaClO2������Һ�����¶ȵ���38��ʱ����������NaClO2��3H2O�����¶ȸ���38��ʱ����������NaClO2����������ͼ��ʾ��NaClO2���ܽ�����ߣ��벹���NaClO2��Һ���Ƶ�NaClO2�IJ������裺
�� ���� ����ϴ�ӣ��ܸ��
��4��NaClO2���ʿɷֽ�ΪNaClO3��NaCl��ȡ����������ǰ���NaClO2�����������Һ���ֱ�������FeSO4��Һ��Ӧʱ������Fe2+�����ʵ�����ͬ���ӵ����غ�ĽǶȽ�����ԭ���� ��
ClO2�ܲ��ȶ������������ƣ�������ˮ���յõ�ClO2��Һ��Ϊ�ⶨ������Һ��ClO2�ĺ���������������ʵ�飺
����1��ȷ��ȡClO2��Һ10.00 mL��ϡ�ͳ�100.00 mL��������ȡV1 mL�������뵽��ƿ�У�
����2������������pH��2.0������������KI���壬����Ƭ�̣�
����3���������ָʾ������c mol��L��1 Na2S2O3��Һ�ζ����յ㣬����Na2S2O3��ҺV2 mL������֪2 Na2S2O3 + I2 ��Na2S4O6 + 2NaI��
��5���жϵζ��յ������ ��ԭClO2��Һ��Ũ��Ϊ g / L���ò����е���ĸ����ʽ��ʾ����
��1��2KClO3+ H2C2O4 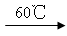 K2CO3+CO2��+2ClO2��+H2O ��2�֣�
K2CO3+CO2��+2ClO2��+H2O ��2�֣�
��2���¶ȼơ�������ˮԡ��������2�֣�
��3�������ᾧ�������ȹ��ˡ�������2�֣�
��4������������ԭ��Ӧ�����е���ת���غ㣬��Ӧ������������Ԫ������Ϊ��1�ۣ�����FeSO4��ʧȥ�ĵ�������ȣ����ĵ�FeSO4����Ҳ��ȡ���������������2�֣�
��5���ӵ����һ��ʱ����Һ����ɫͻȻ����ɫ��Ϊ��ɫ���Ұ���Ӳ��仯��2�֣�����135cV2/V1��2�֣�
���������������1��A�з�Ӧ������K2CO3��ClO2��CO2��,����60�棬����غͲ��ᷴӦ����̼��ء�������̼���������Ⱥ�ˮ����Ӧ����ʽΪ��2KClO3+H2C2O4=K2CO3+CO2��+2ClO2��+H2O;
��2��Ҫ�����¶ȱ���ʹ���¶ȼƲ����¶ȣ���60��ʱ��Ӧ�Ƶã�Ӧ��ˮԡ���ȣ������ձ�����ˮԡ�������������ȵ��۵�ϵͣ�Ϊ�ռ��������ȣ�Ӧ�ڽϵ��¶��½��У�����Ӧ�ò��ñ�ˮԡ���ʴ�Ϊ����ˮԡ�� �ձ�����ˮԡ�������¶ȼƣ�
��3��������ϢNaClO2������Һ�����¶ȵ���38��ʱ����������NaClO2��3H2O�����¶ȸ���38��ʱ����������NaClO2����������ͼ��ʾ��NaClO2���ܽ�����ߣ������¶������ܽ����������벹���NaClO2��Һ���Ƶ�NaClO2�IJ�������Ϊ�����ᾧ�ͳ��ȹ��ˡ�
��4������������ԭ��Ӧ�����е���ת���غ㣬��Ӧ������������Ԫ������Ϊ��1�ۣ�����FeSO4��ʧȥ�ĵ�������ȣ����ĵ�FeSO4����Ҳ��ȡ�
��5���жϵζ��յ������Ϊ�ζ��յ�ʱ��I2��ȫ��Ӧ����Һ����ɫ��Ϊ��ɫ���ʴ�Ϊ����ɫ��Ϊ��ɫ�Ұ���Ӳ��仯��
��6����ԭClO2��Һ��Ũ��Ϊx��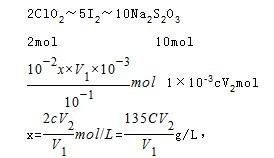
���㣺���⿼����ʵ�鷽������ƣ�ͬʱ����ѧ���������⡢����������������ȷ���ʵ������ǽⱾ��ؼ����ѶȽϴ�



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
��ˮFeCl3���غ�ɫ�����׳��⣬100������ʱ��������ҵ�ϳ������л��ϳɴ�����ʵ���ҿ�������װ��(�г�������ȥ)�Ʊ����ռ���ˮFeCl3��
(1)װ��A�з�Ӧ�����ӷ���ʽΪ______________________��
(2)װ��F�����ӵ��Լ�Ϊ________��
(3)����b������Ϊ________��װ��B������Ϊ___________��
(4)ʵ��ʱӦ�ȵ�ȼA���ľƾ��ƣ���Ӧһ������ٵ�ȼD���ľƾ��ƣ�ԭ��Ϊ____________________________________��
(5)��Ӧ������жװ��ǰ��������еIJ�����__________��
(6)Ϊ�������ò�Ʒ���Ƿ���FeCl2���ɽ�������ʵ�飺ȡE���ռ��IJ�����������ˮ�ܽ⣬��������Һ�м���һ���Լ������Լ�Ϊ________(�����)��
��Fe�� ��KSCN��Һ ������KMnO4��Һ ��NaOH��Һ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
��ͼ��ʾ����ʵ���ҽ��а��������Ʊ�������ʵ������װ�ã����̶ֹ�װ��δ������ 
��l������װ��װ�ú���Ҫ����A��Eװ�õ������ԣ�������ǣ� ���� ��Ȼ����A���۲쵽E��������ð�����ƿ��ƾ��ƻ��ɿ�˫�֣�E�е�����ˮ���γ�˵��װ�����������á�
��2��װ��B��ʢ���Լ��� ��
��3����ȼC���ƾ��ƣ��رյ��ɼ�2�����ɼ�1���ӷ�Һ©���ų�Ũ��ˮ����û��ƿ�й����رշ�Һ©�����Ժ�Ƭ�̣�װ��C�к�ɫ������죬װ��E����Һ����ִ������ݣ�ͬʱ��������������E���ݳ�Һ����������ֱ�������������д����C�з�����Ӧ�Ļ�ѧ����ʽ ��
��4����C�й���ȫ�����ɫ�رյ��ɼ�1�������ƿ��ƾ��ƣ�����ȴ����C�й�������������Ӧǰ��������Ϊ16g����Ӧ����ع�����������2��4g��ͨ������ȷ���ù������ijɷ��� ���û�ѧʽ��ʾ����
��5���ڹرյ��ɼ�1���ɼ�2�������������F�У��ܿ췢��װ��F�в������̣�ͬʱ����G����ҺѸ�ٵ�������F�С�д���������̵Ļ�ѧ����ʽ ��
Ѹ�ٲ���������ԭ���� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
ij��ѧʵ��С���ͬѧΪ̽���ͱȽ�SO2����ˮ��Ư���ԣ���������µ�ʵ��װ�á�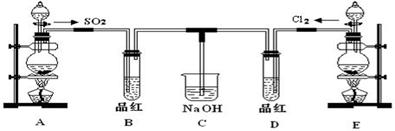
��1��ʵ������װ��A�Ʊ�SO2��ijͬѧ��ʵ��ʱ���ִ�A�ķ�Һ©��������©����Һ��δ���£�����Ϊԭ������� ��
��2��ʵ������װ��E�Ʊ�Cl2���䷴Ӧ�Ļ�ѧ��ѧ����ʽΪ��
MnO2+4HCl��Ũ�� MnCl2+Cl2��+2H2O������6 mol��HCl�μӷ�Ӧ����ת�Ƶĵ�������Ϊ ��
MnCl2+Cl2��+2H2O������6 mol��HCl�μӷ�Ӧ����ת�Ƶĵ�������Ϊ ��
��3���ٷ�Ӧ��ʼһ��ʱ��۲쵽B��D�����Թ��е�Ʒ����Һ���ֵ������ǣ�B�� ��D�� ��
��ֹͣͨ����,�ٸ�B��D�����Թֱܷ���ȣ������Թ��е�����ֱ�ΪB�� ��D�� ��
��4����һ��ʵ��С���ͬѧ��ΪSO2����ˮ����Ư���ԣ�����Ϻ��Ư���Կ϶����ǿ�����ǽ��Ƶõ�SO2��Cl2��1��1ͬʱͨ�뵽Ʒ����Һ�У����������ɫЧ���������������������������������ԭ���û�ѧ����ʽ��ʾ��
��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
��ϴ�������ǹ辧Ƭ��������Ҫ����֮һ����Ƭ��ѧ��ϴ����ҪĿ���dz�ȥ��Ƭ�������ʣ���ijЩ�л�����Σ�������Si��SiO2�۳��ȣ������õĻ�ѧ��ϴ���иߴ�ˮ���л��ܼ���˫��ˮ��Ũ�ᡢǿ��ȡ�����ȥ����������ͨ����һ��Ũ�ȵ�HF��Һ�����������½���Ƭ����1�������ӡ��������ڹ�Ƭ�����γɽ������ε����棬���ӹ��̫��������ա���������ͨ����NaOH��Na2SiO3�Ȼ����Һ��75��90�淴Ӧ25��35 min��Ч�����á��ش���������
��.��1��д����Ƭ����Ӧ�����ӷ���ʽ ���Ե�������1990�껯ѧ��Seidel�����һ�ֵĵ绯ѧģ�ͣ���ָ��Si��NaOH��Һ�ķ�Ӧ��������Si��OHһ��Ӧ������SiO44һ��Ȼ��SiO44һѸ��ˮ������H4SiO4�����ڴ�ԭ��������Ӧ��������Ϊ ��
��2����У��ѧ��ȤС��ͬѧ��Ϊ��֤Seidel�������Ƿ���ȷ���������ʵ�飺
| | ʵ����ʵ |
| ��ʵһ | ˮ������600��ʱ��ʹ��ĩ״�軺���������ų������� |
| ��ʵ�� | ʢ���ڲ���ʯӢ�����еĴ�ˮ��ʱ��Է�ĩ״��ԭ����ʴ���á� |
| ��ʵ�� | ��ͨ���������е�ˮ�����дӲ������ܳ������ļ���ʹ��ĩ״�������л����ܽ⡣ |
| ��ʵ�� | ��Ұ�����ýϸ߰ٷֱȵĹ�����������Ca(OH)2��NaOH�����ź����գ��ɾ��ҷų�H2�� |
| ��ʵ�� | 1g��0.036mo1��Si��20mL����lgNaOH��0.025mol������Һ��С�ļ��ȣ���Ԥ�ȣ����ռ���Լ1700mL H2���ܽӽ�����ֵ��1600mL���� |
 2MgO��Si��ͬʱ�и���Ӧ������2Mg��Si
2MgO��Si��ͬʱ�и���Ӧ������2Mg��Si Mg2Si��Mg2Si������Ѹ�ٷ�Ӧ����SiH4(����)��SiH4�ڳ�������һ�ֲ��ȶ����ֽ�����塣��ͼ�ǽ���Mg��SiO2��Ӧ��ʵ��װ�ã�
Mg2Si��Mg2Si������Ѹ�ٷ�Ӧ����SiH4(����)��SiH4�ڳ�������һ�ֲ��ȶ����ֽ�����塣��ͼ�ǽ���Mg��SiO2��Ӧ��ʵ��װ�ã�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
��Ϥʵ������������ȷ����ʵ����������û�ѧʵ���ǰ�ᡣ
��1�������й�ʵ�������ʵ����ʵ����������ȷ���� (�����)��
A��ʵ������Ũ����Ӧ��������ɫϸ��ƿ�У���������ͼ��ʾ��ǩ |
| B����50mL��Ͳ��ȡ5��6mLŨ���� |
| C���к͵ζ�ʵ��ʱ����ƿϴ�Ӹɾ����ñ�Һ��ϴ����ע�����Һ |
| D�������Ȼ�̼��ȡ��ˮ�еĵ⣬��Һʱ�л���ӷ�Һ©�����¶˷ų� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
ij��ѧС�������ͼʵ��װ�ã��г�װ����ȥ���Ʊ�Cl2��̽��������������ʣ�
��1����װ��A�еĹ���ҩƷΪKClO3����Ӧ��ÿ����3mol Cl2 ʱת�Ƶ��ӵ����ʵ���
Ϊ ��
��2��װ��B���ܳ�ȥ�����е��Ȼ��⣬���ܼ��ʵ�������װ��C�Ƿ�����������C�з���������B�н��۲쵽�������� ��
��3��װ��C����������֤�����Ƿ����Ư���ԣ�l����ʪ�����ɫ���������Ӧ��������ʷֱ��� �� ��
��4�����װ��D��E��Ŀ���DZȽ��ȡ��塢��ķǽ����ԡ��������˵���ȡ��塢��ǽ�����ǿ����ʵ����������� ��
��5���û�ѧ����ʽ˵��װ��F������ ��
��6����ͬѧ�����װ��F�е��Լ��ɸ���������Na2SO3��Һ����ͬѧ����˼������Ϊ�˷������С��������ӷ���ʽ��������Ϊ�����е�ԭ�� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
�������Ϸ��֣�N2��ȡ�����в�ͬ������
a����������������NH3��ԭCuO�Ƶô�����N2�ͻ���ͭ��
�ⷽ��������NaNO2��NH4Cl��Ũ��Һ�Ƶ�
c������������������ͨ�����ȵ�ͭ���Ƶýϴ���N2
����ʵ�����й�ѡ������¼�����������ȡN2
��1������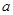 ������N2ʱ����Ҫ�İ�������ʯ�Һ�Ũ��ˮ��ԭ����ȡ�����˲������������е�____________����A��B��C������ͬ����NH3�ķ�������Ҫ��ȡ���ռ�N2������ѡ�õ���������___________��
������N2ʱ����Ҫ�İ�������ʯ�Һ�Ũ��ˮ��ԭ����ȡ�����˲������������е�____________����A��B��C������ͬ����NH3�ķ�������Ҫ��ȡ���ռ�N2������ѡ�õ���������___________��
��2��д��b�����з�Ӧ�Ļ�ѧ����ʽ_________________________
��3������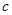 ������ȡN2��������һ�����к����������� ______________________
������ȡN2��������һ�����к����������� ______________________
��4��������N2�����������У�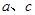 �������ʹ��Խ��Խ�ܵ����ǵĹ�ע�����ַ�����
�������ʹ��Խ��Խ�ܵ����ǵĹ�ע�����ַ�����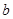 ������ȣ�����Խ������____________��
������ȣ�����Խ������____________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
ʵ������ϡ������ͭ��Ӧ���Ʊ�NO���壬������ã���)װ�ã���ƿ�ڼ���ϡ�����ͭƬ����Ҫʱ�ɼ��ȣ�,ʵ��Ч����ʮ�����룬��Ϊ�ӹ۲쵽������������֤����Ӧ������NO����������ˣ���)װ�ã���Ƥ���¶�����ͭ˿Ȧ������������ʵ����Դﵽ�����Ч��������Ҫ��ش��������⣺
��1���â�װ����ʵ��ʱ��ʵ����������ڹ۲쵽��ƿ���� ������������֤����Ӧ������NO���ռ�NO�ܷ���ƿ�������ſ��������� ����ܡ�������
��2���â�װ����ʵ��ʱ�����йز���������ȫ��
�ٽ���Һ©���Ļ�������U�ιܵ�B��ܿ�ע��ϡ���ᣬһֱע�� Ϊֹ��
a���պý�ûͭ˿�¶� b���պý�ûͭ˿�в� c�� ������������Һ��䲻���п�϶
�ڹرջ������þƾ��ƶ�U�ιܵ�A����ȣ��� ʱ����ȥ�ƾ��ơ�
��3���ڣ�2����ʵ���У�
��ʲô����·�Ӧ�Զ�ֹͣ�� ��
�δ��۲쵽��ɫ��NO���壿 ��
������ٽ���Һ©���Ļ������������ڷ�Һ©���й۲쵽��Щ��������
�� ��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com