
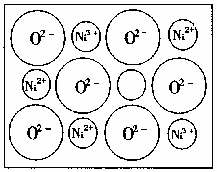
(2)��Ȼ�;����˹��Ʊ��ľ��嶼���ڸ���ȱ�ݣ�������ij��NiO�����оʹ�����ͼ��ʾ��ȱ�ݣ�һ��Ni2����ȱ��������������Ni3����ȡ�����������Գ��ֵ����ԣ�ֻ�ǻ�������Ni��O�ı�ֵ�����˱仯��ij��������Ʒ���ΪNi0.97O���Լ���þ�����Ni3����Ni2����������֮�ȡ�

ͼ3��26
������(1)![]() ��ѡȡ����в�ͬ��������ѡȡ��������С���ظ��ṹ��λ����������Ϊ�о�����Ҳ��ѡȡ������
��ѡȡ����в�ͬ��������ѡȡ��������С���ظ��ṹ��λ����������Ϊ�о�����Ҳ��ѡȡ������

(2)�ⷨһ������غ㷨��?
��1 mol Ni0.97O�к�Ni3+ x mol��Ni2+(0.97��x) mol���ݵ����ԣ�?
3xmol+2��(0.97-x) mol=2��1 mol?
x=0.06��Ni2+Ϊ(0.97-x) mol=0.91 mol������֮��ΪNi3+��Ni2+=0.06��0.91=6��91?
�ⷨ��������������������ѧʽ�Ŵ�100��ΪNi97O100��
�������⣺ÿ3��Ni2+�൱��2��Ni3+���������?
����ȥ��Ni2+�������� Ni3+������ȱ
��������3����������2����������1
��������9����������6����������3
��ÿ97��Ni�к���Ni3+6����Ni2+91��,?
��![]()
�ⷨ�����з����鷨������a��Ni3+��b��Ni2+��c��O��
 ��ã�a��b��6��91
��ã�a��b��6��91
�ⷨ�ģ��Ѿ��忴��Ni2O3��NiO���ֳɷ���ɡ���Ni2O3 xmol��NiO y mol��������Ni0.97O��1 mol��
 ���
���![]()
��![]()
�𰸣���1��![]() g��cm-3
g��cm-3
��2��Ni3+��Ni2+��������֮��Ϊ6��91



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ��֪ʶ����������ѵ������������ѧ ���ͣ�038
(1)��ѧ�̲���ͼʾ��NaCl����ṹ��������ά�ռ�������������壮NiO(������)����Ľṹ��NaCl��ͬ��Ni2+�����ڽ�O2���ĺ˼����Ϊa��10��8cm�����㾧����ܶ�(��֪NiO��Ħ������Ϊ74.7g/mol)

(2)��Ȼ�ĺ;����˹��Ʊ��ľ��嶼���ڸ���ȱ�ݣ�������ij��NiO�����оʹ�����ͼ��ʾ��ȱ�ݣ�һ��Ni2+��ȱ����������Ni2+������Ni3+��ȡ�������������Գʵ����ԣ�����������Ni��O�ı�ֵ�������˱仯��ij��������Ʒ���Ϊ��Ni0.97O���Լ���þ�����Ni3+��Ni2+��������֮�ȣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 (1)��ѧ�̲���ͼʾ��NaCl����ṹ��������ά�ռ�����õ��������塣NiO(������)����ṹ��NaCl��ͬ��Ni2+�����ڽ���O2���ĺ˼����Ϊa��10��8cm������NiO������ܶȡ�(��֪NiO��Ħ������Ϊ��74��7g��mol��1)
(1)��ѧ�̲���ͼʾ��NaCl����ṹ��������ά�ռ�����õ��������塣NiO(������)����ṹ��NaCl��ͬ��Ni2+�����ڽ���O2���ĺ˼����Ϊa��10��8cm������NiO������ܶȡ�(��֪NiO��Ħ������Ϊ��74��7g��mol��1)
(2)��Ȼ�ĺʹ��˹����嶼���ڸ���ȱ�ݣ�������ij��NiO�����оʹ�������ͼ��ʾ��ȱ�ݣ�һ��Ni2+��ȱ����������Ni2+������Ni3+�����档���������Գʵ����ԡ�����������Ni��O�ı�ֵȴ�����˱仯��ij��������Ʒ���ΪNi0.97O���Լ���þ�����Ni3+��Ni2+��������֮�ȡ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
(1)��ѧ�̲���ͼʾ��NaCl����ṹ��������ά�ռ�����õ��������塣 NiO��������������Ľṹ��NaQ��ͬ��Ni2+�����ڽ�O2-�ĺ˼����Ϊa10-8cm������NiO������ܶȣ���֪NiO��Ħ������Ϊ74��7g��mol-1����
(2)��Ȼ�ĺ;����˹��Ʊ��ľ��嶼���ڸ���ȱ�ݣ�������ij��NiO�����оʹ�������ͼ��ʾ��ȱ�ݣ�һ��Ni2+��ȱ����������Ni2+������Ni3+��ȡ�������������Գʵ����ԣ�����������Ni��O�ı�ֵȴ�����˱仯��ij��������Ʒ���ΪNio970,�Լ���þ�����Ni3+��Ni2+��������֮�ȡ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��ͬ���� ���ͣ�������

�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com