
��֪���������ʹ���Ը��������Һ��ɫ����ѧ��Ӧ����ʽΪ��
5SO2 + 2KMnO4 + 2H2O �� K2SO4 + 2MnSO4 + 2H2SO4
����ͼװ������֤Ũ������ľ̿�ڼ��������·�Ӧ�IJ����к���SO2��CO2

(1) д��Ũ�����ľ̿�ڼ��������·�����Ӧ�Ļ�ѧ����ʽ��________________
(2) ʵ��ʱ����Ӧ����������Ӧ��_________��ͨ�룻 ��________������ʢ�г���ʯ��ˮ��ʵ��װ��(�� ��a����b�� ���)��
(3) Bƿ��������_______________��
(4) ֤����������к��ж�����̼�������� ____________��____________��

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ��2016-2017ѧ�갲��ʡ����ɽ�и߶���ѧ����ĩ���Ի�ѧ�Ծ��������棩 ���ͣ������
(1)��CH4����ԭNOx�������������������Ⱦ�����磺
CH4(g)��4NO2(g)===4NO(g)��CO2(g)��2H2O(g)�� ��H����574 kJ��mol��1
CH4(g)��4NO(g)===2N2(g)��CO2(g)��2H2O(g) ��H����1 160 kJ��mol��1
���ñ�״����4.48 L CH4��ԭNO2����N2����Ӧ��ת�Ƶĵ�������Ϊ________ (�����ӵ�������NA��ʾ)���ų�������Ϊ________kJ��
(2)��֪��C3H8(g) == CH4(g)��HC��CH(g)��H2(g)����H1����156.6 kJ��mol��1
CH3CH=CH2(g) == CH4(g)��HC��CH(g) ��H2����32.4 kJ��mol��1
����ͬ�����£���ӦC3H8(g) === CH3CH=CH2(g)��H2(g)�Ħ�H��______kJ��mol��1��
(3)�����ڸ�������ˮ������Ӧ�ķ���ʽΪCH4(g)��H2O(g) CO(g)��3H2(g)���������ʵ�ȼ�����������±�����֪1 mol H2O(g)ת��Ϊ1 mol H2O(l)ʱ�ų�44.0 kJ������д��CH4��H2O�ڸ����·�Ӧ���Ȼ�ѧ����ʽ�� _______________________________��
CO(g)��3H2(g)���������ʵ�ȼ�����������±�����֪1 mol H2O(g)ת��Ϊ1 mol H2O(l)ʱ�ų�44.0 kJ������д��CH4��H2O�ڸ����·�Ӧ���Ȼ�ѧ����ʽ�� _______________________________��
���� | ȼ����(kJ��mol��1) |
H2(g) | ��285.8 |
CO(g) | ��283.0 |
CH4(g) | ��890.3 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2016-2017ѧ�������ʡĵ�����и�һ��ѧ�ڿ�ѧ��⻯ѧ�Ծ��������棩 ���ͣ������
��1��ʵ���������Ľ����Ʊ�����___________�У�ȡ��ʱ�õ�����������Ʒ��С��������Ƭ����ֽ��____________, ʣ�����Ӧ_______________����һС����Ͷ�뵽����ͭ��Һ�У���Ӧ�����ӷ���ʽΪ______________________________________���۲쵽������Ϊ_____________(��д���)��
a���Ƹ���Һ�����Ĵ��ζ� b�����ڳ���һ��������С��
c����Һ������ɫ�������� d����Һ���к�ɫ��������
��2��ʵ�����������Ļ�ѧ��Ӧ����ʽΪ_________________________________������Ϊ�ж����壬��������������Һ����β������������ɸ����ӷ���ʽ____________�� ��ԭ���������ڹ�ҵ��______________��
��3���������ʼ��������ᷴӦ���������ռ���Һ��Ӧ����________________________________
a. Al b. Mg c. CH3COONH4 d. NaHCO3 e. Al2O3
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2016-2017ѧ�������ʡĵ�����и�һ��ѧ�ڿ�ѧ��⻯ѧ�Ծ��������棩 ���ͣ�ѡ����
100ml 1mol/L��AlCl3��Һ��100ml 3.5mol/L��NaOH��Һ��ϣ��õ�����Ϊ( )
A. 7.8g B. 0g C. 9.1 g D. 3.9g
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2016-2017ѧ�������ʡĵ�����и�һ��ѧ�ڿ�ѧ��⻯ѧ�Ծ��������棩 ���ͣ�ѡ����
���и�������������;�Ĺ�ϵ����ȷ����( )
A. �������ƣ������� B. �ռ ����θ������һ��ҩ��
C. С�մ� ���ͷ���Ҫ�ɷ� D. ������ ��ˮ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2016-2017ѧ�갲��ʡ�����и�һ��ѧ����ĩ���Ի�ѧ�Ծ��������棩 ���ͣ�ѡ����
����������Ϊ���ʣ�ѡ���г����ʵķ�����ȷ����
A. Cu ��CuO�� ����ϡ������Һ��Ȼ�����ϴ�Ӹ���
B. CO2 ( SO2 ) ͨ������Na2 CO3 ��Һ
C. CO2 ��O2 �� ͨ�����ȵ�ͭ��
D. NO2 ��NO�� ͨ��ˮϴ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2016-2017ѧ�갲��ʡ�����и�һ��ѧ����ĩ���Ի�ѧ�Ծ��������棩 ���ͣ�ѡ����
�й�SiO2������ε�˵����ȷ����
A. ˮ�ࡢʯӢ�������մɾ��ǹ����β�Ʒ
B. ��̫���ܵ�ذ����õ��Ǹߴ���SiO2
C. �ֻ���������ͨ�����ɷ���ͬ
D. SiO2������HF�ᷴӦ������NaOH��Ӧ����SiO2Ϊ����������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ʡ��ɳ��2016-2017ѧ���һ��ѧ����ĩ���Ի�ѧ�Ծ� ���ͣ�ѡ����
�������ӷ���ʽ��ȷ����
A. ����������ˮ�Ʊ������Cl2+H2O�T2H++Cl-+ClO-
B. ̼�����ϡ���ᷴӦ��CO32-+2H+=H2O+CO2��
C. �������Ͱ�ˮ��Ӧ��Al3++3OH-��Al(OH)3��
D. Na��H2O��Ӧ��2Na+2H2O=2Na++2OH-+H2��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2017�츣��ʡ�����е���У�����ڶ������Ͽ������ۻ�ѧ�Ծ��������棩 ���ͣ������
��(Sn)��һ����Ҫ�Ľ������������������������ϡ��뵼�塣��Ԫ��λ�����ڱ���5���ڣ���IVA�塣
�����ڻ������г����Ļ��ϼ������֣�+2��_________��
��������������Ҳ����NaOH��Һ��Ӧ�����ɵ��ζ��Ǻ�+2�۵�����д������NaOH��Һ��Ӧ�����ӷ���ʽ_____________________________��
����ͼ�ǹ�ҵұ�����Ļ������̣�
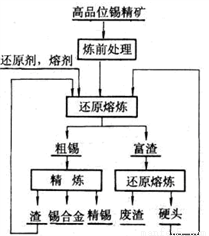
����ǰ����������������Ҫ����������ͭ��Ǧ��������黯��ڿ����б��ռ����̣�ʹ�����Ԫ��ת��Ϊ�ӷ���SO2��As2O3����д����Ż�����FeAsS2���������ɺ���ɫ����Ļ�ѧ����ʽ______________�����ɵ������ù�����NaOH��Һ���գ�����Һ�е�Ũ�����ĺ������������_______
�ڻ�ԭ�����ķ�Ӧԭ��Ϊ��SnO2(s)+2CO(g) Sn(s)+2CO2(g)��һ���¶��£��ڹ̶��ݻ����ܱ������У�SnO2��CO��Ӧ��ƽ��������������ƽ��Ħ������Ϊ37.6�����¶��µ�ƽ�ⳣ��K=____________
Sn(s)+2CO2(g)��һ���¶��£��ڹ̶��ݻ����ܱ������У�SnO2��CO��Ӧ��ƽ��������������ƽ��Ħ������Ϊ37.6�����¶��µ�ƽ�ⳣ��K=____________
�۴�������Ҫ����Fe��Cu��Pb��Sb�����������õ�⾫��������þ���������SnSO4��Һ�����Һ���������ӵ�Դ��______��������֪�����������к���Sb��Cu��PbSO4�ȣ���������������缫��ӦʽΪ________________________________________��
�ܾ����������������ԭ����������Ӳͷ��һ�����е�Ԫ����________��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com