
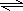 Al��OH��3+3H+����Һ�����ԣ�
Al��OH��3+3H+����Һ�����ԣ�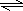 Al��OH��3+3H+��Al2O3��
Al��OH��3+3H+��Al2O3��

 ��У��ʦ������ҵ���Ӻ����Ծ�ϵ�д�
��У��ʦ������ҵ���Ӻ����Ծ�ϵ�д� ȫ�̽��ϵ�д�
ȫ�̽��ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
A.pH=12�İ�ˮ��Һ��pH=2��������Һ��������
c(![]() )��c(Cl-)��c(OH-)��c(H+)
)��c(Cl-)��c(OH-)��c(H+)
B.Ũ�Ⱦ�Ϊ0.1 mol��L-1�����������Һ������������Һ��������
c(Na+)��c(![]() )��c(
)��c(![]() )��c(H+)��c(OH-)
)��c(H+)��c(OH-)
C.Ũ�Ⱦ�Ϊ0.1 mol��L-1��С�մ���Һ���ռ���Һ��������
c(Na+)+c(H+)=2c(![]() )+c(OH-)
)+c(OH-)
D.Ũ�Ⱦ�Ϊ0.1 mol��L-1�Ĵ�����Һ������������Һ��������
c(Na+)=c(CH3COO-)��c(OH-)=c(H+)
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
A.pH=12�İ�ˮ��Һ��pH=2��������Һ��������
c(![]() )��c(Cl-)��c(OH-)��c(H+)
)��c(Cl-)��c(OH-)��c(H+)
B.Ũ�Ⱦ�Ϊ0.1 mol��L��1�����������Һ������������Һ��������
c(Na+)��c(![]() )��c(
)��c(![]() )��c(H+)��c(OH-)
)��c(H+)��c(OH-)
C.Ũ�Ⱦ�Ϊ0.1 mol��L��1��С�մ���Һ���ռ���Һ��������
c(Na+)��c(H+)��![]() )��c(OH-)��c(
)��c(OH-)��c(![]() )
)
D.Ũ�Ⱦ�Ϊ0.1 mol��L-1�Ĵ�����Һ������������Һ��������
c(Na+)��c(CH3COO-)��c(OH-)��c(H+)
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��8�֣�����Ũ�Ⱦ�Ϊ0.1 mol/L��������Һ�������ᡢ�ڴ��ᡢ���������ơ����Ȼ�李��ݴ���李�������李���������泥��ఱˮ����ش��������⣺
��1���١��ڡ��ۡ���������Һ����ˮ�������H+Ũ���ɴ�С��˳���ǣ�����ţ�
��
��2���ܡ��ݡ��ޡ��ߡ���������Һ��NH4+Ũ���ɴ�С��˳���ǣ�����ţ�
��
��3�����ۺܵ͢������Ϻ��Һ�и�����Ũ���ɴ�С��˳��������������
�� ����������
��4����֪t�棬KW��1��10��13����t�棨�>����<�������� 25�档��t��ʱ��pH��11��NaOH��Һa L��pH��1��H2SO4����Һb L��ϣ����Ի�Ϻ���Һ����ı仯���������û����Һ��pH��2����a��b�� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011-2012���Ͻ���ʡ����һ�и߶���ѧ�ڵ�һ�ζο���ѧ�Ծ� ���ͣ���ѡ��
��8�֣�����Ũ�Ⱦ�Ϊ0.1 mol/L��������Һ�������ᡢ�ڴ��ᡢ���������ơ����Ȼ�李��ݴ���李�������李���������泥��ఱˮ����ش��������⣺
��1���١��ڡ��ۡ���������Һ����ˮ�������H+Ũ���ɴ�С��˳���ǣ�����ţ�
��
��2���ܡ��ݡ��ޡ��ߡ���������Һ��NH4+Ũ���ɴ�С��˳���ǣ�����ţ�
��
��3�����ۺܵ͢������Ϻ��Һ�и�����Ũ���ɴ�С��˳��������������
�� ����������
��4����֪t�棬KW��1��10��13����t�棨�>����<�������� 25�档��t��ʱ��pH��11��NaOH��Һa L��pH��1��H2SO4����Һb L��ϣ����Ի�Ϻ���Һ����ı仯���������û����Һ��pH��2����a��b�� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011-2012���Ͻ���ʡ�߶���ѧ�ڵ�һ�ζο���ѧ�Ծ� ���ͣ�ѡ����
��8�֣�����Ũ�Ⱦ�Ϊ0.1 mol/L��������Һ�������ᡢ�ڴ��ᡢ���������ơ����Ȼ�李��ݴ���李�������李���������泥��ఱˮ����ش��������⣺
��1���١��ڡ��ۡ���������Һ����ˮ�������H+Ũ���ɴ�С��˳���ǣ�����ţ�
��
��2���ܡ��ݡ��ޡ��ߡ���������Һ��NH4+Ũ���ɴ�С��˳���ǣ�����ţ�
��
��3�����ۺܵ͢������Ϻ��Һ�и�����Ũ���ɴ�С��˳��������������
�� ����������
��4����֪t�棬KW��1��10��13����t�棨�>����<�������� 25�档��t��ʱ��pH��11��NaOH��Һa L��pH��1��H2SO4����Һb L��ϣ����Ի�Ϻ���Һ����ı仯���������û����Һ��pH��2����a��b�� ��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com