
��10�֣��������ᆳ�Ȼ����������Ȼ����ǹ�ҵ�����Ȼ���ij��÷�����Cl2��CCl4�dz��õ��Ȼ������磺
 Na2O��Cl2��2NaCl��O2
Na2O��Cl2��2NaCl��O2
CaO��Cl2��CaCl2��O2
SiO2��2CCl4��SiCl4��2COCl2
Cr2O3��3CCl4��2CrCl3��3COCl2
��ش��������⣺
�� Cr2O3��CrCl3��Cr��Ϊ��3�ۣ�д��Cr3���Ļ�̬�����Ų�ʽ ��
�� CCl4������Cԭ�Ӳ�ȡ �ӻ��ɼ���
�� COCl2�׳ƹ�����������Cԭ�Ӳ�ȡsp2�ӻ��ɼ����������ӵĽṹʽ�� ������̼��ԭ��֮�乲�ۼ��� ������ţ���
a��2���Ҽ� b��2���м� c��1���Ҽ���1���м�
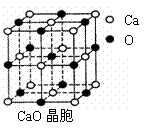
�� CaO����������ͼ��ʾ��CaO������Ca2������λ��Ϊ ��CaO�����NaCl�������������з�ʽ��ͬ���侧���ֱܷ�Ϊ��CaO��3 401kJ•mol��1��NaCl��786kJ•mol��1���������߾����ܲ������Ҫԭ���� ��
 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��10�֣��������ᆳ�Ȼ����������Ȼ����ǹ�ҵ�����Ȼ���ij��÷�����Cl2��CCl4�dz��õ��Ȼ������磺
Na2O��Cl2��2NaCl��O2
CaO��Cl2��CaCl2��O2
SiO2��2CCl4��SiCl4��2COCl2
Cr2O3��3CCl4��2CrCl3��3COCl2
��ش��������⣺
��1��Cr2O3��CrCl3��Cr��Ϊ��3�ۣ�д��Cr3���Ļ�̬�����Ų�ʽ ��
��2��CCl4������Cԭ�Ӳ�ȡ �ӻ��ɼ���
��3��COCl2�׳ƹ�����������Cԭ�Ӳ�ȡsp2�ӻ��ɼ����������ӵĽṹʽ�� ������̼��ԭ��֮�乲�ۼ��� ������ţ���
a��2���Ҽ� b��2���м� c��1���Ҽ���1���м�
��4��CaO����������ͼ��ʾ��CaO������Ca2������λ��Ϊ ��CaO�����NaCl�������������з�ʽ��ͬ���侧���ֱܷ�Ϊ��CaO��3 401kJ•mol��1��NaCl��786kJ•mol��1���������߾����ܲ������Ҫԭ���� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��14�֣��������ᆳ�Ȼ����������Ȼ����ǹ�ҵ�����Ȼ���ij��÷�����Cl2��CCl4�dz��õ��Ȼ������磺
Na2O��Cl2��2NaCl��O2
CaO��Cl2��CaCl2��O2
SiO2��2CCl4��SiCl4��2COCl2
Cr2O3��3CCl4��2CrCl3��3COCl2
��ش��������⣺
��д������ԭ�ӽṹʾ��ͼΪ ���ͬ���ڵ�����Ԫ�صĻ�̬ԭ�����������������ԭ����ͬ��Ԫ����____________����Ԫ�ط��ţ�������һ�ֽ����ľ����ṹ��ͼ��ʾ���þ����к��н���ԭ�ӵ���ĿΪ________��
�� CCl4������Cԭ�Ӳ�ȡ �ӻ��ɼ���
�� COCl2�׳ƹ�����������Cԭ�Ӳ�ȡsp2�ӻ��ɼ����������ӵĽṹʽ�� ������̼��ԭ��֮�乲�ۼ��� ������ţ�
a��2���Ҽ� b��2���м� c��1���Ҽ���1���м�
�� CaO��������ͼ��ʾ��CaO������Ca2������λ��Ϊ ��CaO�����NaCl�������������з�ʽ��ͬ���侧���ֱܷ�Ϊ��CaO��3401kJ•mol��1��NaCl��786kJ•mol��1���������߾����ܲ������Ҫԭ���� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2010-2011ѧ�꽭��ʡ�������У�߶���ѧ�����е��л�ѧ�Ծ� ���ͣ������
��14�֣��������ᆳ�Ȼ����������Ȼ����ǹ�ҵ�����Ȼ���ij��÷�����Cl2��CCl4�dz��õ��Ȼ������磺
Na2O��Cl2��2NaCl��O2
CaO��Cl2��CaCl2��O2
SiO2��2CCl4��SiCl4��2COCl2
Cr2O3��3CCl4��2CrCl3��3COCl2
��ش��������⣺
��д������ԭ�ӽṹʾ��ͼΪ ���ͬ���ڵ�����Ԫ�صĻ�̬ԭ�����������������ԭ����ͬ��Ԫ����____________����Ԫ�ط��ţ�������һ�ֽ����ľ����ṹ��ͼ��ʾ���þ����к��н���ԭ�ӵ���ĿΪ________��
�� CCl4������Cԭ�Ӳ�ȡ �ӻ��ɼ���
�� COCl2�׳ƹ�����������Cԭ�Ӳ�ȡsp2�ӻ��ɼ����������ӵĽṹʽ�� ������̼��ԭ��֮�乲�ۼ��� ������ţ�
a��2���Ҽ� b��2���м� c��1���Ҽ���1���м�
�� CaO��������ͼ��ʾ��CaO������Ca2������λ��Ϊ ��CaO�����NaCl�������������з�ʽ��ͬ���侧���ֱܷ�Ϊ��CaO��3401kJ?mol��1��NaCl��786kJ?mol��1���������߾����ܲ������Ҫԭ���� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2010�꽭��ʡ�߶��ڶ�ѧ����ĩ���Ի�ѧ�Ծ� ���ͣ������
��10�֣��������ᆳ�Ȼ����������Ȼ����ǹ�ҵ�����Ȼ���ij��÷�����Cl2��CCl4�dz��õ��Ȼ������磺
Na2O��Cl2��2NaCl��O2
CaO��Cl2��CaCl2��O2
SiO2��2CCl4��SiCl4��2COCl2
Cr2O3��3CCl4��2CrCl3��3COCl2
��ش��������⣺
��1��Cr2O3��CrCl3��Cr��Ϊ��3�ۣ�д��Cr3���Ļ�̬�����Ų�ʽ ��
��2��CCl4������Cԭ�Ӳ�ȡ �ӻ��ɼ���
��3��COCl2�׳ƹ�����������Cԭ�Ӳ�ȡsp2�ӻ��ɼ����������ӵĽṹʽ�� ������̼��ԭ��֮�乲�ۼ��� ������ţ���
a��2���Ҽ� b��2���м� c��1���Ҽ���1���м�
��4��CaO����������ͼ��ʾ��CaO������Ca2������λ��Ϊ ��CaO�����NaCl�������������з�ʽ��ͬ���侧���ֱܷ�Ϊ��CaO��3 401kJ•mol��1��NaCl��786kJ•mol��1���������߾����ܲ������Ҫԭ���� ��

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012�콭��ʡ�������У�߶���ѧ�����е��л�ѧ�Ծ� ���ͣ������
��14�֣��������ᆳ�Ȼ����������Ȼ����ǹ�ҵ�����Ȼ���ij��÷�����Cl2��CCl4�dz��õ��Ȼ������磺
Na2O��Cl2��2NaCl��O2
CaO��Cl2��CaCl2��O2
SiO2��2CCl4��SiCl4��2COCl2
Cr2O3��3CCl4��2CrCl3��3COCl2
��ش��������⣺
��д������ԭ�ӽṹʾ��ͼΪ ���ͬ���ڵ�����Ԫ�صĻ�̬ԭ�����������������ԭ����ͬ��Ԫ����____________����Ԫ�ط��ţ�������һ�ֽ����ľ����ṹ��ͼ��ʾ���þ����к��н���ԭ�ӵ���ĿΪ________��

�� CCl4������Cԭ�Ӳ�ȡ �ӻ��ɼ���
�� COCl2�׳ƹ�����������Cԭ�Ӳ�ȡsp2�ӻ��ɼ����������ӵĽṹʽ�� ������̼��ԭ��֮�乲�ۼ��� ������ţ�
a��2���Ҽ� b��2���м� c��1���Ҽ���1���м�

�� CaO��������ͼ��ʾ��CaO������Ca2������λ��Ϊ ��CaO�����NaCl�������������з�ʽ��ͬ���侧���ֱܷ�Ϊ��CaO��3401kJ•mol��1��NaCl��786kJ•mol��1���������߾����ܲ������Ҫԭ���� ��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com