
(1)��ͬѧ�ķ����ǣ�����Ʒ�ܽ⣬�ӹ����Ȼ�����Һ������ϴ�ӣ�ȡ������ɣ������ù���n g����������̼���Ƶ���������Ϊ_____________����Ca2+��Ba2+����ʹ![]() ������ȫ����ʹ���Ȼ�����Һ���Ȼ�����Һ���õĽ�����и��ߵľ�ȷ�ȣ�ԭ����__________________________________��
������ȫ����ʹ���Ȼ�����Һ���Ȼ�����Һ���õĽ�����и��ߵľ�ȷ�ȣ�ԭ����__________________________________��
(2)��ͬѧ�ķ�����ͼ��ʾ��
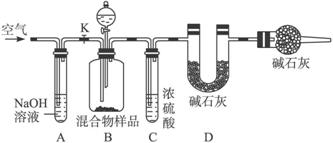
���ݸ�ʵ�鷽������ͬѧ��ʵ���б���ȷ�ⶨ��������_____________�����������Ʒ��ַ�Ӧ��ȫʱ������ͨ�������Ŀ����_____________�����У�װ��A��������_____________��
װ��E��������__________________________________________________________��
 ��һ����ͬ���ɽ�����ϵ�д�
��һ����ͬ���ɽ�����ϵ�д� ������Ӧ���ϵ�д�
������Ӧ���ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 ���о�NO2��SO2��CO�ȴ�����Ⱦ����Ĵ���������Ҫ���壮
���о�NO2��SO2��CO�ȴ�����Ⱦ����Ĵ���������Ҫ���壮| Q2-Q1 |
| 2 |
| Q2-Q1 |
| 2 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2015��㶫ʡ�����и߶���ѧ��ĩ���Ի�ѧ�Ծ��������棩 ���ͣ�ʵ����
��1��ijѧϰС����0.80mol/L��Ũ�ȵ��ռ���Һ�ⶨδ֪Ũ�ȵ����ᡣ
�ٵζ�����ͼ��ʾ���� �ζ���ʢװ��Ũ�ȵ�����������Һ����ס����ҡ�����

���õζ��ķ������ⶨ�����Ũ�ȣ�ʵ������������ʾ��
|
ʵ���� |
����HCl��Һ�����(mL) |
����NaOH��Һ�����(mL) |
|
1 |
20.00 |
23.00 |
|
2 |
20.00 |
23.10 |
|
3 |
20.00 |
22.90 |
��δ֪�����Ũ��Ϊ��������λ��Ч���֣�_______________��
�����в�����ʹ����õ������Ũ��ƫ�͵���__________��
A��ʢװ����Һ����ƿ��ˮϴ��δ����
B���ζ�ǰ����ʽ�ζ��ܼ�������ݣ��ζ���������ʧ
C����ʽ�ζ���������ˮϴ����δ�ñ�����������Һ��ϴ
D������ʽ�ζ��ܵĿ̶�ʱ���ζ�ǰ���Ӱ�Һ����ʹ����ζ����Ӷ���
��2��ij����С��Ϊ�˲ⶨij�Ȼ���(SrCl2)��Ʒ�Ĵ��ȣ���������·�������ȡ1.0 g��Ʒ�ܽ�������ˮ�У������м��뺬AgNO3 2.38 g��AgNO3��Һ(��Һ�г�Cl���⣬����������Ag����Ӧ���ɳ���������)��Cl������ȫ��������Ȼ���ú�Fe3������Һ��ָʾ������0.2 mol��L��1��NH4SCN����Һ�ζ�ʣ���AgNO3��ʹʣ���Ag����AgSCN��ɫ��������ʽ�������Բⶨ�Ȼ�����Ʒ�Ĵ��ȡ�
��ش��������⣺
���жϵζ��ﵽ�յ��������_______________________________________________��
�ڿ���Ag����Fe3������������Һ�еĴ�����ʽ����ʵʩ�ζ�����Һ�Գ�_____(ѡ����ԡ��������ԡ����ԡ�)Ϊ�ˡ�
�����յ㵽��֮ǰ�ĵζ������У����ֳ����������������Ag�����費�Ͼ���ҡ����ƿ�������ʹn(Cl��)�IJⶨ���________(ѡ�ƫ�ߡ�����ƫ�͡�����Ӱ�족)��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
ij����С��Ϊ��̽���Ҷ��������ǿ�����Ҷ������ȷֽ������������ʵ�顣
(1)��pH��ֽ�ⶨ�����ʵ���Ũ�ȵ����ᡢ�Ҷ��ᡢ������Һ��pH�������Һ��pH�ֱ�Ϊa��b��c����a��b��c��
�ٲⶨ������ҺpH�ķ�����________________________________________________��
�������������������ǿ������˳����__________________________________________��
(2)��һ�������Ҷ�������Թ��У�����ͼװ�ý���ʵ�顣

��ּ���һ��ʱ�������װ����U�ι����������Һ�壬��װ���г���ʯ��ˮ����ǡ�����Ӧ��ɺ�����װ����U�ι������������ˮ����ͭ����ˮ����ͭ������
�ٸ�������ʵ����ʵ���Ʋ��Ҷ���ֽ������һ����__________________________________��
����װ�õ�������________________________________________________________��
(3)���ݶ�װ���Ʋ⣬�Ҷ���ֽ�IJ����л�������___________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
���о�NO2��SO2��CO�ȴ�����Ⱦ����Ĵ���������Ҫ���塣
��1����֪��2SO2(g) + O2(g)![]() 2SO3(g) ��H= ��Q1 kJ��mol-1
2SO3(g) ��H= ��Q1 kJ��mol-1
2NO(g) + O2(g)![]() 2NO2(g) ��H= ��Q2kJ��mol-1
2NO2(g) ��H= ��Q2kJ��mol-1
��ӦNO2(g) + SO2(g)![]() SO3(g) + NO(g) �Ħ�H= kJ��mol-1��
SO3(g) + NO(g) �Ħ�H= kJ��mol-1��
 ��2��һ�������£���NO2��SO2�������1:2�����ܱ������з���
��2��һ�������£���NO2��SO2�������1:2�����ܱ������з���
������Ӧ�� �����������Ӧƽ��ʱNO2��NO�����Ϊ1:3��
��ƽ�ⳣ��K�� ��
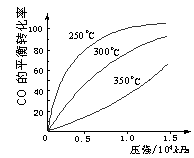
��3��CO�����ںϳɼ״�����Ӧ����ʽΪ��
CO��g��+2H2��g��![]() CH3OH��g����CO�ڲ�ͬ�¶��µ�
CH3OH��g����CO�ڲ�ͬ�¶��µ�
ƽ��ת������ѹǿ�Ĺ�ϵ����ͼ��ʾ���÷�Ӧ��H 0
���>���� <������
����֪������һ�ֶ�Ԫ���ᣬ�������ƣ�NaHC2O4����Һ�����ԡ�
��1�������ӷ���ʽ����Na2C2O4��Һ�Լ��Ե�ԭ�� ��
��2�������£��Ƚ�0.1 mol��L-1NaHC2O4��Һ�и�������Ũ�ȵĴ�С��ϵ ��
��ij����С��Ϊ��̽����BaSO4�ܽ�ȣ��ֱ�����BaSO4���룺a. 5ml ˮ;b. 40 ml 0.2 mol��L-1��Ba(OH)2��Һ��c. 20ml 0.5 mol��L-1��Na2SO4��Һ��d. 40ml 0.1 mol��L-1��H2SO4��Һ�У��ܽ������͡�
��1�����ϸ���Һ�У���Ũ���ɴ�С��˳��Ϊ ��
A.b>a>c>d B.b>a>d>c C.a>d>c>b D.a>b>d>c
��2��ijͬѧȡͬ������Һb����Һdֱ�ӻ�ϣ�������Һ��pHֵΪ ��������Һ�����Ϊ���ǰ����Һ�����֮�ͣ���
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012-2013ѧ������ʡ��Ϫһ�и������ϣ��¿���ѧ�Ծ���10�·ݣ��������棩 ���ͣ������
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com