
��֪��һ��NaCl�����к���4��Na+��4��Cl-����һ��NaCl�������Ϊ8a3cm3��ʵ�����п���NaCl����ȷ�ⶨ�����ӵ�������NA�����䲽�����£�
�ٽ�����NaClϸ�������ȷ��ȡm g NaCl���岢ת�Ƶ���������A�У�
���õζ�����A�����мӱ��������������ӱ���A�����Ŀ̶ȣ������NaCl��������V cm3�����㰢���ӵ�����
��ش��������⣺
��1���������A�������ʹ��____________������ţ���
A.��Ͳ B.�ձ� C.����ƿ D.�Թ�
��2����ʵ���еζ���Ӧѡ____________�ζ��ܣ����ʽ��ʽ����ʽ������ԭ��____________
____________ ��
��3���ܷ���ˮ���汽____________������____________
____________ ��
��4��NA����ʽΪ____________ ��
����������Ŀ�Ĺؼ���ȷ���NaCl�����������NaCl�����Dz�������״�ҿ������п�϶������Ŀ��ʵ�鲽��֪����һ��������NaCl����һ���ݻ�������ƿ�У�Ȼ�������NaCl�������ݵı���������NaCl�����V(NaCl)=V(����ƿ)-V����)����4)V cm3 NaCl����n(Na+)=n(Cl-)=V/(8a3��4)=V/2a3������ÿ1��Na+��Cl-������=m g��(V/2a3)=2a3 m/V g����585 g����NA= 58.5 g/2a3m/Vg = 58.5V/2a3m ��
�𰸣���1)C
��2)��ʽ��ʽ�ζ��ܵ������ܽ�����Σ�3)����NaCl����ˮ���ܲ��NaCl����������4)NA=58.5V/2a3m



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ������ʡ������ѧ2012�������ѧ�ڵ�һ���¿���ѧ���� ���ͣ�022
������й���ļ������Σ���������������ظ���λ����Ϊ��������ѧ�̲���NaCl����ṹ��ΪNaCl��һ����������֪FexO����ľ����ṹΪNaCl�ͣ����ھ���ȱ�ݣ�x��1��ʵ����FexO������ܶ�Ϊ5.71 g/cm3�������ı߳�Ϊ4.28��10��5 m��NaCl�ľ�����ͼ��ʾ��
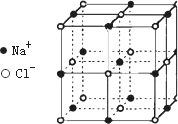
(1)��FexO��X�ľ�����ֵ(��ȷ��0.01)Ϊ________��������FeԪ��ֻ�У�2�ͣ�3�ۣ�����Fe2+��Fe3+�������У�Fe2+��ռ����(��С����ʾ����ȷ��0.001)Ϊ________
(2)�˾���Ļ�ѧʽΪ________
(3)��������O2�������������ȵ�Fe2+��Fe3+��Χ�ɵļ��ι�����________
(4)�����У�Fe���Ӽ��������Ϊ________cm
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
������й���ļ������Σ���������������ظ���λ����Ϊ��������ѧ�̲���NaCl����ṹ��ΪNaCl��һ����������֪FexO����ľ����ṹΪNaCl�ͣ����ھ���ȱ�ݣ�x<1��ʵ����FexO������ܶ�Ϊ5.71g/cm3�������ı߳�Ϊ4.28��10-5m��NaCl�ľ�����ͼ��ʾ��
(1)��FexO��X�ľ�����ֵ(��ȷ��0.01)Ϊ ��������FeԪ��ֻ��+2��+3�ۣ�����Fe2+��Fe3+ �������У�Fe2+��ռ����(��С����ʾ����ȷ��0.001)Ϊ
(2)�˾���Ļ�ѧʽΪ
(3)��������O2-�����������ȵ�Fe2+��Fe3+��Χ�ɵļ��ι�����
(4)������,Fe���Ӽ��������Ϊ cm
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012������ʡ������ѧ������һ���¿���ѧ�Ծ� ���ͣ������
������й���ļ������Σ���������������ظ���λ����Ϊ��������ѧ�̲���NaCl����ṹ��ΪNaCl��һ����������֪FexO����ľ����ṹΪNaCl�ͣ����ھ���ȱ�ݣ�x<1��ʵ����FexO������ܶ�Ϊ5.71g/cm3�������ı߳�Ϊ4.28��10-5m��NaCl�ľ�����ͼ��ʾ��
(1)��FexO��X�ľ�����ֵ(��ȷ��0.01)Ϊ ��������FeԪ��ֻ��+2��+3�ۣ�����Fe2+��Fe3+ �������У�Fe2+��ռ����(��С����ʾ����ȷ��0.001)Ϊ
(2)�˾���Ļ�ѧʽΪ
(3)��������O2-�����������ȵ�Fe2+��Fe3+��Χ�ɵļ��ι����� (4)������,Fe���Ӽ��������Ϊ cm
(4)������,Fe���Ӽ��������Ϊ cm
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011-2012ѧ������ʡ������һ���¿���ѧ�Ծ� ���ͣ������
������й���ļ������Σ���������������ظ���λ����Ϊ��������ѧ�̲���NaCl����ṹ��ΪNaCl��һ����������֪FexO����ľ����ṹΪNaCl�ͣ����ھ���ȱ�ݣ�x<1��ʵ����FexO������ܶ�Ϊ5.71g/cm3�������ı߳�Ϊ4.28��10-5m��NaCl�ľ�����ͼ��ʾ��
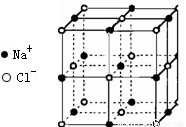
(1)��FexO��X�ľ�����ֵ(��ȷ��0.01)Ϊ ��������FeԪ��ֻ��+2��+3�ۣ�����Fe2+��Fe3+ �������У�Fe2+��ռ����(��С����ʾ����ȷ��0.001)Ϊ
(2)�˾���Ļ�ѧʽΪ
(3)��������O2-�����������ȵ�Fe2+��Fe3+��Χ�ɵļ��ι�����
(4)������,Fe���Ӽ��������Ϊ cm
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com