
 ���IJ����Ͷ�Ϊ
���IJ����Ͷ�Ϊ


| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
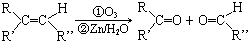
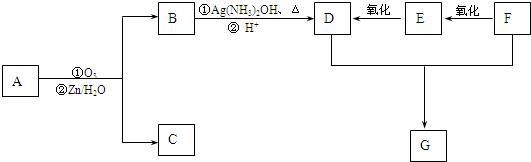
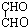 +4Cu��OH��2
+4Cu��OH��2| �� |
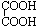 +2Cu2O��+4H2O
+2Cu2O��+4H2O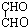 +4Cu��OH��2
+4Cu��OH��2| �� |
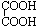 +2Cu2O��+4H2O
+2Cu2O��+4H2O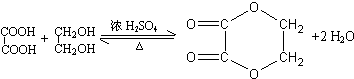
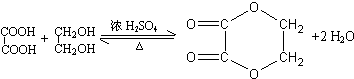
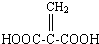
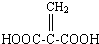
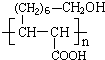
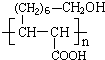
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
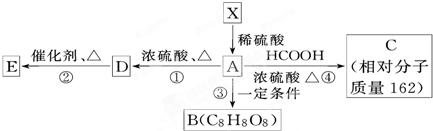
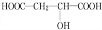 +HCOOH
+HCOOH| Ũ���� |
| �� |
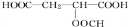 +H2O
+H2O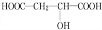 +HCOOH
+HCOOH| Ũ���� |
| �� |
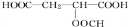 +H2O
+H2O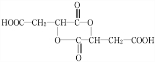

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
(1)C16H20���ӵIJ����ͶȦ�=���������̼̼ԭ�Ӽ�Ĺ��õ��Ӷ���Ϊ_____________��
(2)Cx�ɿ������������IJ����C60��̼̼ԭ�Ӽ�Ĺ��õ��Ӷ���Ϊ____________��
(3)��ijȲ��������̼̼ԭ�Ӽ�Ĺ��õ��Ӷ���Ϊ8�������ķ���ʽ��__________����������к���4��������ṹ��ʽ��__________��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com