

���� ��������Ʒ�к������������������������������ϡ�����ˮ�ܽⷴӦ�õ��Ȼ������Ȼ����������Һ������250.00ml��Һ���õζ�����ȡ25.00ml��Һ������ˮ�����������ӣ����������ˮ���������ӣ�����ϴ�ӵõ������������������յõ�����ɫ����Ϊ��������
��1���������к��������������������ϡ���ᷴӦ�õ��Ȼ������Ȼ����������ݵ���غ��Ԫ���غ���ƽ���ӷ���ʽ��
��2��������Ϊ����һ���������Һ����������һ���������Һ���õ��IJ����������ձ�����ͷ�ιܡ���������250 mL����ƿ���ݴ˴��⣻
��3����ˮ���������ӷ���������ԭ��Ӧ�����������Ӻ������ӣ�
��4����������ķ�����֪����ɫ��������ƣ�
��5�����������������a1g�������������պ�������������a2g����������������Ϊa2g-a1g��������Ԫ�ص�����Ϊ$\frac{112}{160}$����a2g-a1g�����ݴ˼�����Ʒ����Ԫ�ص�����������
��� �⣺��1���������к��������������������ϡ���ᷴӦ�õ��Ȼ������Ȼ�������������Һ����Ԫ�صĴ�����̬ΪFe2+��Fe3+�������ӷ�Ӧ���̴�+7�۽�Ϊ+2�ۣ�����+2����Ϊ+3�ۣ����ݵ���غ��Ԫ���غ��д�����ӷ���ʽΪMnO4-+5Fe2++8H+=Mn2++5Fe3++4H2O��
�ʴ�Ϊ��Fe2+��Fe3+��1��5��8H+��1��5��4H2O��
��2��������Ϊ����һ���������Һ����������һ���������Һ���õ��IJ����������ձ�����ͷ�ιܡ���������250 mL����ƿ��
�ʴ�Ϊ����������250 mL����ƿ��
��3����ˮ���������ӷ���������ԭ��Ӧ�����������Ӻ������ӣ���Ӧ���ӷ���ʽΪ2Fe 2++Br2=2Fe 3++2Br-��
�ʴ�Ϊ��2Fe 2++Br2=2Fe 3++2Br-��
��4����������ķ�����֪����ɫ����Ϊ��������
�ʴ�Ϊ����������
��5�����������������a1g�������������պ�������������a2g����������������Ϊa2g-a1g������ag��Ʒ����Ԫ�ص�����Ϊ$\frac{112}{160}$����a2g-a1g����$\frac{250}{25}$��������Ʒ����Ԫ�ص���������Ϊ$\frac{\frac{112}{160}����a{\;}_{2}g-a{\;}_{1}g����\frac{250}{25}}{ag}$��100%=$\frac{7��{a}_{2}-{a}_{1}��}{a}$��100%��
�ʴ�Ϊ��$\frac{7��{a}_{2}-{a}_{1}��}{a}$��100%��
���� ���⿼����Һ���ơ����ӷ���ʽ����ƽ����ʵ�������������ʵ�鷽�������ۡ���ѧ����ȣ��Ѷ��еȣ�����ⶨԭ���ǽ���Ĺؼ����Ƕ���ѧ֪ʶ���ۺ����ã���Ҫѧ��������ʵ�Ļ���������֪ʶ������������������


 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | �٢� | B�� | �٢� | C�� | �ڢ� | D�� | �٢ڢ� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | Na+�ĵ����Ų�ͼ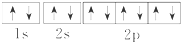 | |
| B�� | Na+�Ľṹʾ��ͼ��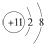 | |
| C�� | ��̬Naԭ�ӵĵ����Ų�ʽ��1s22s22p53s2 | |
| D�� | ��̬Naԭ�ӵļ۵����Ų�ʽ��3s1 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ������̼ | B�� | �� | C�� | �ռ���Һ | D�� | NaCl |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ��H1����H2����H3 | B�� | ��H1����H3����H2 | C�� | ��H3����H2����H1 | D�� | ��H2����H1����H3 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ������þ��ɫ���������Ȼ����Һ��Mg��OH��2+2NH${\;}_{4}^{+}$=Mg2++2NH3•H2O | |
| B�� | ����һ������Һˮ�⣺HPO${\;}_{4}^{2-}$+H2O?PO${\;}_{4}^{3-}$+H3O+ | |
| C�� | ���AlCl3��Һ��2Al${\;}_{3}^{+}$+6Cl-+6H2O$\frac{\underline{\;���\;}}{\;}$2Al��OH��3��+3H2��+3Cl2�� | |
| D�� | ͭƬ�ӵ�������̼���ӵ縺�������������Һ��Cu+2H+$\frac{\underline{\;���\;}}{\;}$Cu2++H2�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
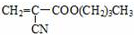 ����ϳɷ���֮һ��·�����£�
����ϳɷ���֮һ��·�����£�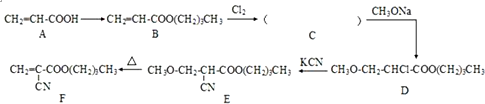
 ��
���鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com