
����Ŀ��úȼ���ŷŵ�������![]() ��Ҫ��
��Ҫ��![]() ��
��![]() ��
��![]() �γ����ꡢ��Ⱦ�������������������ش��������⣺
�γ����ꡢ��Ⱦ�������������������ش��������⣺

(1)����![]() ��������ɵõ��Ϻõ�Ч������֪���з�Ӧ��
��������ɵõ��Ϻõ�Ч������֪���з�Ӧ��
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
��Ӧ![]()
![]()
![]()
![]() ��
��![]() ______ ��
______ ��
(2)���ð�ˮ�����������տɵõ����ʣ�����ͬ���ʵ�����![]() ��
��![]() ����ˮ������Һ��
����ˮ������Һ��![]() ______
______ ![]() ����ĸ���
����ĸ���![]() ��
��
A.![]() /span>
/span>![]()
C.![]()
![]()
(3)�����ڽϸ��¶Ⱦ���ͼ1�����ѳ�![]() �����Ƶ�
�����Ƶ�![]() ��
��
���������ŵ�������� ______ ��
������������![]() �ĵ缫��Ӧʽ�� ______ ��
�ĵ缫��Ӧʽ�� ______ ��
����֪�����£�![]() ���ѳ�
���ѳ�![]() ���Ƶõ�
���Ƶõ�![]() ���
���![]() ��
��![]() ��Һ����
��Һ����![]() ��
��![]() ��Һ��ϣ������û����Һ��
��Һ��ϣ������û����Һ��![]() ,��
,��![]() ��Һ��
��Һ��![]() ��Һ�������Ϊ ______
��Һ�������Ϊ ______ ![]() ��ʹ��Һ��
��ʹ��Һ��![]() ����Ӧ������Һ��
����Ӧ������Һ��![]() ______
______ ![]() ��
��
(4)һ�������£���![]() ��NiO��
��NiO��![]() ���������������·�Ӧ����ȼú�����е���ӦΪ��
���������������·�Ӧ����ȼú�����е���ӦΪ��![]()
![]() ����������ͬ��������ͬʱ��
����������ͬ��������ͬʱ��![]() ��ת�����淴Ӧ�¶ȵı仯��ͼ2�������Ǵ����ļ۸����أ�ѡ�� ______ Ϊ�÷�Ӧ�Ĵ�����Ϊ����
��ת�����淴Ӧ�¶ȵı仯��ͼ2�������Ǵ����ļ۸����أ�ѡ�� ______ Ϊ�÷�Ӧ�Ĵ�����Ϊ����![]() ѡ�����
ѡ�����![]() ��
��
![]()
![]()
![]()
ѡ��ô����������ǣ� ______ ��
ij����С����ѡ��Ĵ�������![]() ʱ���о���
ʱ���о���![]() ��
��![]() �ֱ�Ϊ1��1��3��1ʱ��
�ֱ�Ϊ1��1��3��1ʱ��![]() ת���ʵı仯���
ת���ʵı仯���![]() ͼ
ͼ![]() ��ͼ3�б�ʾ
��ͼ3�б�ʾ![]() ��
��![]() ��1�ı仯����Ϊ ______ ��
��1�ı仯����Ϊ ______ ��
���𰸡�![]() BD
BD ![]()
![]() 10��1
10��1 ![]() c
c ![]() ������ʱ������Խϵ��¶ȿɻ�ýϸߵ�
������ʱ������Խϵ��¶ȿɻ�ýϸߵ�![]() ת���ʣ��Ӷ���Լ��Դ a
ת���ʣ��Ӷ���Լ��Դ a
��������
��1�����ݸ�˹���ɿ�֪��+��-�ۼ��õ���Ӧ��
��2����ͬ���ʵ�����SO2��NH3����ˮ��Ӧ������������泥������Һ�е���غ�������غ�����ж�ѡ�
��3��������������ԭ��Ӧ����������������Ӧ����ʾ��ͼ��֪��������������õ��ӵõ�SO42-��
������������������������ʧȥ��������SO3��O2��
��ˮ�����ӻ�����KW=10-14��pH=9��Ba��OH��2��Һ������������Ũ��Ϊ0.00001mol/L��pH=4��H2SO4��Һ��������Ũ��Ϊ0.0001mol/L�������û��ҺΪ���ԣ����ǡ����ȫ��Ӧ�������ӵ����ʵ����������������ӵ����ʵ������ݴ���ʽ�����Ba��OH��2 ��Һ�� H2SO4 ��Һ������ȵ�ֵ������Q=c��Ba2+��c��SO42-��������Һ��c��SO42-����1.0��10-5molL-1����Ӧ������Һ�� c��Ba2+����ֵ��
��4��Fe2O3������ʱ������Խϵ��¶ȿɻ�ýϸߵ�SO2ת���ʣ��Ӷ���Լ��Դ�� n��CO����n��SO2��Խ���������ת����Խ��
��1����֪![]()
![]()
![]()
����ݸ�˹���ɿ�֪![]() ���õ���Ӧ
���õ���Ӧ![]() ��
��
�ʴ�Ϊ��![]() ��
��
��2����ͬ���ʵ�����![]() ��
��![]() ����ˮ������Ӧ������������泥���Ӧ�����ӷ���ʽΪ��
����ˮ������Ӧ������������泥���Ӧ�����ӷ���ʽΪ��![]() ����Һ�д��ڵ���غ㣺
����Һ�д��ڵ���غ㣺![]() ��
��![]() ��D��ȷ��C������Һ�д��������غ㣺
��D��ȷ��C������Һ�д��������غ㣺![]() ����ϵ���غ�������غ��֪
����ϵ���غ�������غ��֪![]() ������A����B��ȷ��
������A����B��ȷ��
�ʴ�Ϊ��BD��
��3������������ԭ��Ӧ����������������Ӧ����ʾ��ͼ��֪��������������õ��ӵõ�![]() ������������������������ʧȥ��������
������������������������ʧȥ��������![]() ��
��![]() ��
��
��������������õ��ӣ��ʴ�Ϊ��![]() ��
��
�������缫��Ӧ����ʽΪ��![]() ��
��
�ʴ�Ϊ��![]() ��
��
�������£�ˮ�����ӻ�����![]() ��
��![]() ��
��![]() ��Һ������������Ũ��Ϊ
��Һ������������Ũ��Ϊ![]() ��
��![]() ��
��![]() ��Һ��������Ũ��Ϊ
��Һ��������Ũ��Ϊ![]() �������û��ҺΪ���ԣ����ǡ����ȫ��Ӧ�����У�
�������û��ҺΪ���ԣ����ǡ����ȫ��Ӧ�����У�![]() ����
����![]() ��Һ��
��Һ��![]() ��Һ������ֱ�ΪaL��bL������
��Һ������ֱ�ΪaL��bL������![]() ��a��
��a��![]() ��1��
��1��![]() �������£�
�������£�![]() ����ʹ��Һ��
����ʹ��Һ��![]() �����
�����![]() ��֪��Һ��Ӧ����
��֪��Һ��Ӧ����![]() ��
��
�ʴ�Ϊ��10��1�� ![]() ��
��
��4��Fe2O3������ʱ������Խϵ��¶ȿɻ�ýϸߵ�![]() ת���ʣ��Ӷ���Լ��Դ����ѡFe2O3��������
ת���ʣ��Ӷ���Լ��Դ����ѡFe2O3��������![]() ��
��![]() Խ���������ת����Խ������a��ʾ
Խ���������ת����Խ������a��ʾ![]() ��
��![]() ��1�ı仯���ߣ�
��1�ı仯���ߣ�
�ʴ�Ϊ��c��![]() ������ʱ������Խϵ��¶ȿɻ�ýϸߵ�
������ʱ������Խϵ��¶ȿɻ�ýϸߵ�![]() ת���ʣ��Ӷ���Լ��Դ��a��
ת���ʣ��Ӷ���Լ��Դ��a��


 ͨ��ѧ��Ĭд����ϵ�д�
ͨ��ѧ��Ĭд����ϵ�д� ���ƽ̸�������ѡ����ĩ���100��ϵ�д�
���ƽ̸�������ѡ����ĩ���100��ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�������£���NaOH��Һ�ζ�H2C2O4��Һ����Һ��-lg[c(H+)/c(H2C2O4)]��-lgc(HC2O4-)��-lg[c(H+)/c(HC2O4-)]��-lgc(C2O42-)��ϵ��ͼ��ʾ������˵���������( )

A. Ka1(H2C2O4)=1��10��2
B. �ζ�������,��pH=5ʱ��C(Na��)��3C(HC2O4-)>0
C. ��1 mol/L��H2C2O4��Һ�м���������Ũ�ȵ�NaOH��Һ����ȫ��Ӧ��������
D. ��0.1 mol/L��H2C2O4��Һ�м�ˮϡ�ͣ�C(HC2O4-)/C(H2C2O4)��ֵ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�������ƾ��壨Na2MoO42H2O����һ����������ȴˮϵͳ�Ľ�����ʴ������ҵ�������⾫����Ҫ�ɷ��Dz�����ˮ��MoS2���Ʊ������Ƶ�����;����ͼ��ʾ��
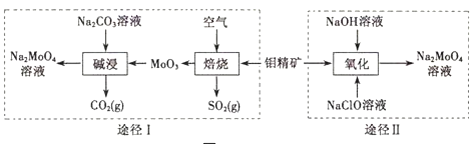
��1������ͬ�����ɽ������Ԫ���Ǻ˷�Ӧ��ȼ�ϰ��İ������ϣ�ﯺϽ��ڸ�������ˮ������Ӧ����������������ﯿ����������������մɣ����й���ﯡ�������ﯵ������У���ȷ����______������ţ�
A ﯺϽ�ȴ�ﯵ��۵�ߣ�Ӳ��С
B ��������մ������������ǽ�������
C ��һ������ͨ������������ﯻ����һ��������ͨ·
��2����;��I���ʱ������Ӧ�����ӷ���ʽΪ______��
��;��������ʱ������Ӧ�����ӷ���ʽΪ______��
��3���������������Ƴ����������[��NH4��2MoO4]���������Ʒ�Ӧ����ȡ�������÷�Ӧ������������;��I��������β��һ��ͨ��ˮ�У��õ����εĻ�ѧʽ��______��
��4�������ƺ��¹���������Ļ��Һ����Ϊ̼�ظֵĻ�ʴ���������£�̼�ظ������ֲ�ͬ�����еĸ�ʴ����ʵ������ͼ��
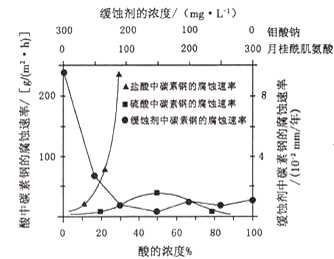
��Ҫʹ̼�ظֵĻ�ʴЧ�����ţ������ƺ��¹����������Ũ�ȱ�ӦΪ______��
�ڵ������Ũ�ȴ���90%ʱ����ʴ���ʼ���Ϊ�㣬ԭ����______��
��5��﮺Ͷ������γɵĶ��ε�ص��ܷ�ӦΪ��xLi+nMoS2![]() Lix��MoS2��n�����طŵ�ʱ��������Ӧʽ�ǣ�______������ʹ����Ϊ50%�ĸõ�أ�����;��I��ʹ���е�Moת��Ϊ�����ƾ��壬�õ�a�˵�Na2MoO42H2O��������ΪM��������Ҫ��������O2Ϊ20%���ڱ���µ����Ϊ______L����x��M��n��ʾ������Ϊ���
Lix��MoS2��n�����طŵ�ʱ��������Ӧʽ�ǣ�______������ʹ����Ϊ50%�ĸõ�أ�����;��I��ʹ���е�Moת��Ϊ�����ƾ��壬�õ�a�˵�Na2MoO42H2O��������ΪM��������Ҫ��������O2Ϊ20%���ڱ���µ����Ϊ______L����x��M��n��ʾ������Ϊ���
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�������Ϊ2 L�ĺ����ܱ������з�����ӦxA(g)��yB(g)![]() zC(g)��ͼ����ʾ200 ��ʱ������A��B��C���ʵ�����ʱ��ı仯��ͼ����ʾ��ͬ�¶���ƽ��ʱC�������������ʼn(A)��n(B)�ı仯��ϵ�������н�����ȷ����
zC(g)��ͼ����ʾ200 ��ʱ������A��B��C���ʵ�����ʱ��ı仯��ͼ����ʾ��ͬ�¶���ƽ��ʱC�������������ʼn(A)��n(B)�ı仯��ϵ�������н�����ȷ����
ͼ�� ͼ��
ͼ��
A. 200 ��ʱ����Ӧ�ӿ�ʼ��ƽ���ƽ������v(B)��0.04mol��L-1��min-1
B. ͼ����֪��ӦxA(g)��yB(g)![]() zC(g)����H>0����a��2
zC(g)����H>0����a��2
C. ����ͼ����ʾ��ƽ��״̬�£�������ϵ�г���He�����´ﵽƽ��ǰv��>v��
D. 200 ��ʱ���������г���2 mol A ��1 mol B���ﵽƽ��ʱ��A�������������0.5
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����1����ѧ��Ӧ����Ϊ�ɼ����Ѻ��¼��γɵĹ��̣���ѧ���ļ������γ�(���)1mol��ѧ��ʱ�ͷ�(������)����������֪����P4O6�ķ��ӽṹ��ͼ��ʾ�����ṩ���»�ѧ���ļ��ܣ�P-P��198kJ��mol-1��P-O��360 kJ��mol-1��O=O��498kJ��mol-1����ӦP4(����)��O2��Ӧ����P4O6���Ȼ�ѧ��Ӧ����ʽΪ____��

��2����(N2H4)����Ϊ�����������ȼ�ϣ���������N2O4��Ӧ����N2��ˮ��������֪��
��N2(g)��2O2(g)�TN2O4(l) ��H1�T-19.5kJ/mol
��N2H4(l)��O2(g)�TN2(g)��2H2O(g) ��H2�T-534.2kJ/mol
д���º�N2O4��Ӧ���Ȼ�ѧ����ʽ_____��
��3����ѧ��ӦN2��3H2![]() 2NH3�������仯��ͼ��ʾ���÷�Ӧ����NH3(l)���Ȼ�ѧ����ʽ��_____��
2NH3�������仯��ͼ��ʾ���÷�Ӧ����NH3(l)���Ȼ�ѧ����ʽ��_____��

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�������ڱ��У�ͬһ����Ԫ�ػ�ѧ�������ơ�ĿǰҲ������ЩԪ�صĻ�ѧ���ʺ��������ڱ������Ϸ������·�����һ����Ԫ���������ƣ����Ϊ�Խ��߹��ݴ���ش�
(1)��ڿ�����ȼ�գ�������________(�ѧʽ����ͬ)�⣬Ҳ��������________��
(2)�������������Ӧ��ˮ����Ļ�ѧʽ��________�������Ի����֤����һ���۵��й����ӷ���ʽΪ_________��
(3)����֪��ӦBe2C��4H2O=2Be(OH)2��CH4������Al4C3������ǿ����Һ��Ӧ�����ӷ���ʽΪ________��
(4)��ѧ��֤ʵ��BeCl2�ǹ��ۻ�������һ����ʵ��֤�����䷽����_______��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����1�����ڷ�Ӧ��2NO(g)��O2(g)![]() 2NO2(g)��������������ͬʱ���ֱ���NO��ƽ��ת�����ڲ�ͬѹǿ(p1��p2)���¶ȱ仯��������ͼ:
2NO2(g)��������������ͬʱ���ֱ���NO��ƽ��ת�����ڲ�ͬѹǿ(p1��p2)���¶ȱ仯��������ͼ:


�ٱȽ�p1��p2�Ĵ�С��ϵ��________��
�����¶����ߣ��÷�Ӧƽ�ⳣ���仯��������________(������С��)��
��2�����ݻ�Ϊ1.00L�������У�ͨ��һ������N2O4��������ӦN2O4(g)![]() 2NO2(g)�����¶����ߣ�����������ɫ����ش��������⣺
2NO2(g)�����¶����ߣ�����������ɫ����ش��������⣺
�ٷ�Ӧ�Ħ�H______0(����ڡ���С�ڡ�)��100��ʱ����ϵ�и�����Ũ����ʱ��仯����ͼ��ʾ����0��60sʱ�Σ���Ӧ����v(N2O4)Ϊ__________________��ƽ��ʱ���������NO2���������Ϊ_______��
��100��ʱ��ƽ�����������Ѹ�ٳ��뺬0.08mol��NO2��0.08mol N2O4 �Ļ�����壬��ʱ���ʹ�ϵv(��)____v���棩��������ڡ��������ڡ�����С�ڡ���
��100��ʱ��ƽ��ı䷴Ӧ�¶�ΪT��c(N2O4)��0.0020mol��L��1��s��1��ƽ�����ʽ��ͣ���10s�ִﵽƽ�⡣
a.T________100��(����ڡ���С�ڡ�)���ж�������____________________
b.��ʽ�����¶�Tʱ��Ӧ��ƽ�ⳣ��K2��д������̣���______
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ������˵������ȷ����
A.![]() �ڳ����¼��ɽ��У�˵������Ӧ�Ƿ��ȷ�Ӧ
�ڳ����¼��ɽ��У�˵������Ӧ�Ƿ��ȷ�Ӧ
B.����β��������ʱ�ķ�Ӧ��![]()
![]() ��ƽ�ⳣ��Ϊ
��ƽ�ⳣ��Ϊ![]() ������Ӧ�ھ��������н��У�ƽ�ⳣ��Ϊ
������Ӧ�ھ��������н��У�ƽ�ⳣ��Ϊ![]() ����
����![]()
C.ij�����ܱ������з�Ӧ��![]()
![]() �Ѵ�ƽ�⣬������ʱ
�Ѵ�ƽ�⣬������ʱ![]() ��ֵ��С
��ֵ��С
D.���º����ܱ������з�Ӧ��![]() �����������ܶȲ��ٸı�ʱ˵����Ӧ�Ѵ�ƽ��
�����������ܶȲ��ٸı�ʱ˵����Ӧ�Ѵ�ƽ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��һ��������������������۷�Ӧʱ��Ϊ�˼�����Ӧ�����Ҳ�Ӱ���������������������������м�������( )
��NaOH(s) ��NH4Cl(s) ��H2O ��CH3COONa(s)
A.�٢�B.�ڢ�C.�ۢ�D.�٢�
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com