
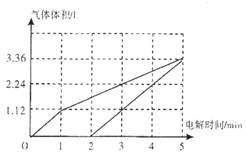
 ���ʴ�Ϊ��
���ʴ�Ϊ�� ��
��
| ||
| 0.1mol |
| 1L |


 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| ʵ���� | HA���ʵ���Ũ�ȣ�mol/L�� | NaOH���ʵ���Ũ�ȣ�mol/L�� | �����Һ��pH |
| �� | 0.20 | 0.20 | pH=a |
| �� | 0.10 | 0.10 | pH=8.00 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ��ʴ���
| ʵ���� | HA���ʵ���Ũ�ȣ�mol/L�� | NaOH���ʵ���Ũ�ȣ�mol/L�� | �����Һ��pH |
| �� | 0.20 | 0.20 | pH=a |
| �� | 0.10 | 0.10 | pH=8.00 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��13�֣�����Ũ��Ϊ0.1 mol?L��1�����ֵ������Һ�� �� Na2CO3 �� NaHCO3 �� NaAlO2 �� CH3COONa �� NaOH
��֪��CO2+3H2O+2AlO2��===2Al(OH)3��+CO32��
��1����������Һ��pH��С�����˳����___ ___�����ţ���
��2����������Һϡ����ͬ�ı���ʱ����pH�仯������____ __�����ţ���
��3�����̼�ᣨH2CO3����Һ��NaAlO2��Һ����д�����ܷ����Ļ�ѧ��Ӧ����ʽ��
��
��4�������£���ijһԪ��HA��NaOH��Һ�������ϣ�������Һ��Ũ�Ⱥͻ�Ϻ�������Һ��pH���±���
ʵ���� | HA���ʵ���Ũ�ȣ�mol/L�� | NaOH���ʵ���Ũ�ȣ�mol/L�� | �����Һ��pH |
�� | 0.20 | 0.20 | pH��a |
�� | 0.10 | 0.10 | pH��8.00 |
�����������ʵ���������Ӽ�����������������a�������Һ��pH����˵��HA��ǿ�ỹ������ ��
����ʵ�����û����Һ����ˮ�������c (OH��)�� mol/L��
����û����Һ��������ʽ��ֵ��Ҫ��д��������������̡�
I��c (Na��)��c (A��)�� ��
��c (OH��)��c (HA)�� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2009��㶫ʡ��������������ɽ��ѧ�߿���ѧ��ģ�Ծ��������棩 ���ͣ������
| ʵ���� | HA���ʵ���Ũ�ȣ�mol/L�� | NaOH���ʵ���Ũ�ȣ�mol/L�� | �����Һ��pH |
| �� | 0.20 | 0.20 | pH=a |
| �� | 0.10 | 0.10 | pH=8.00 |
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com