
��14�֣������»�ѧ��Ӧ��2A��g����B��g�� 2C��g������H<0��
2C��g������H<0��
��1������4 mol A��2 mol B��2 L�������л�ϣ���2 s����C��Ũ��Ϊ0��6 mol/L��������A��ʾ��ƽ����Ӧ����Ϊ____________________________��2sʱ����B��Ũ��Ϊ____________________��
��2������4 mol A��2 mol B�����������������У�һ���¶��´ﵽƽ��״̬��������������ʵ���Ϊ4��2 mol����ʱ�����������C���������Ϊ_____________������ͨ������B���壬��ϵ��A�����ʵ���_________�����������С�����䡱������ҪʹA�����ʵ����ٴﵽ��ԭƽ��״̬��ͬ���ɲ�ȡ�Ĵ�ʩ��_ ������һ�ִ�ʩ���ɣ�
��3����ͼ��һ�������¸÷�Ӧ�����У���ϵ�ڸ�����Ũ�ȵı仯�����t2ʱ���߷����仯��ԭ����_______________________________ ������t4ʱ��B��Ũ������0��l mol��L��1����t5ʱ�̵�����ƽ�⡣����ͼ�л���������Ũ�ȵı仯�����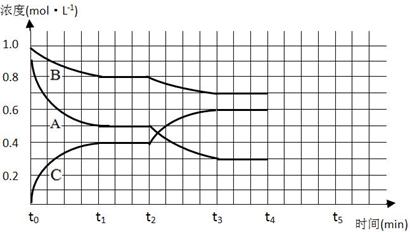
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 �����»�ѧ��Ӧ��2A��g��+B��g��?2C��g������H��0��
�����»�ѧ��Ӧ��2A��g��+B��g��?2C��g������H��0���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
(1)����4 mol A��2 mol B��
(2)����a mol A��b mol B����һ�ܱ������У��ﵽƽ��ʱ���ǵ����ʵ������㣺
n(A)��n(B)=n(C)����A��ת����Ϊ____________��
(3)����4 mol A��2 mol B��������ɱ�ĵ�ѹ�����У�һ���¶��´ﵽƽ��״̬��������������ʵ���Ϊ4.2 mol����ʱ�����������C���������Ϊ____________������ͨ������B���壬��ϵ��A���������____________(�������С�����䡱)����ҪʹA����������ٴﵽ��ԭƽ��״̬��ͬ���ɲ�ȡ�Ĵ�ʩ��________________________��
(4)��ͼ��һ�������¸÷�Ӧ�����У���ϵ�ڸ�����Ũ�ȵı仯�������Ӧ����ƽ��״̬��ʱ����________��t2ʱ���߷����仯��ԭ����_________________������t4ʱ��B��Ũ������0.1 mol��L-1��������ͼ�л���������Ũ�ȵı仯�����

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�����»�ѧ��Ӧ��2A��g����B��g��![]() 2C��g������H<0��
2C��g������H<0��
��1������amolA��bmolB����һ�ܱ������У��ﵽƽ��ʱ���ǵ����ʵ������㣺
n��A����n��B��=n��C������A��ת���ʣ�
��2������4molA��2molB��������ɱ�ĵ�ѹ�����У�һ���¶��´ﵽƽ��״̬��������������ʵ���Ϊ4��2mol����ʱ������������C�����������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2013������ʡ��һ6���¿���ѧ���� ���ͣ�������
�����»�ѧ��Ӧ��2A��g����B��g�� 2C��g������H<0��
2C��g������H<0��
��1������amolA��bmolB����һ�ܱ������У��ﵽƽ��ʱ���ǵ����ʵ������㣺
n��A����n��B��=n��C������A��ת���ʣ�
��2������4molA��2molB��������ɱ�ĵ�ѹ�����У�һ���¶��´ﵽƽ��״̬��������������ʵ���Ϊ4��2mol����ʱ������������C�����������
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com