
��֪:X+Y![]() Z+W
Z+W
(1)Y�ĵ���ʽ��_________________________��
(2)Һ̬Z��W�ĵ������ƣ����ɵ������������ͬ���������ӣ�Һ̬Z�ĵ��뷽��ʽ��_________________________________��
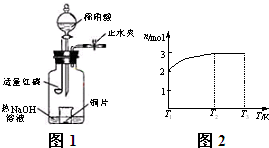
(3)��ͼʾװ���Ʊ�NO����֤�仹ԭ�ԡ���������Ҫ������
a.����ƿ��ע��������NaOH��Һ����ʢ��ͭƬ��С�ձ�����ƿ�С�
b.�ر�ֹˮ�У���ȼ���ף�����ƿ�У����ý�����
c.�����׳��ȼ�գ�һ��ʱ����Һ©�����������ձ��е�������ϡ���ᡣ

�ٲ���c��ȱ�ٵ�һ����Ҫ������_______________________________________��
�ں��׳��ȼ�յIJ�����NaOH��Һ��Ӧ�����ӷ���ʽ�� ___________________________________________________________________��
�۲���c����ϡ������ձ��е�������______________________________________
____________________________________________________________________��
��Ӧ�����ӷ���ʽ��____________________________________________________��
(4)һ���¶��£���1 mol N2O4�����ܱ������У�����ѹǿ���䣬�����¶���T1�Ĺ����У���������ɫ��Ϊ����ɫ���¶���T1�������ߵ�T2�Ĺ����У�������Ϊ��ɫ��������T2������ѹǿ��������Ϊ����ɫ����������ʵ���n���¶�T�仯�Ĺ�ϵ��ͼ��ʾ��

���¶���T1��T2֮�䣬��Ӧ�Ļ�ѧ����ʽ��_________________________��
���¶���T2��T3֮�䣬�����ƽ����Է��������ǣ�����1λС����______________��
��1��![]()
��2��2NH3(I)![]()
![]() +
+![]()
(3)�ٴ�ֹˮ�У�ͨ����������
��P2O5+6OH-![]() 2
2![]() +3H2O
+3H2O
��CuƬ���ܽ⣬����ɫ���ݲ�������Һ����ɫ��Ϊ��ɫ
3Cu+8H++2![]()
![]() 3Cu2++2NO��+4H2O
3Cu2++2NO��+4H2O
(4)��2NO2 ![]() 2NO+O2
2NO+O2
��30.7
��������������֪������֪��XΪ![]() ��WΪH2O����X+Y
��WΪH2O����X+Y![]() Z+W��֪YΪOH-��ZΪNH3����
Z+W��֪YΪOH-��ZΪNH3����
![]() +OH-
+OH-![]() NH3+H2O
NH3+H2O
(2)Һ����NH3����W(H2O)�������ƣ�����ˮ�ĵ��룺2H2O![]() H3O++OH-��Һ���ĵ��뷽��ʽΪ��2NH3(l)
H3O++OH-��Һ���ĵ��뷽��ʽΪ��2NH3(l)![]()
![]() +
+![]() ��
��
(3)�����ڱ�ʵ��Ŀ���ǡ��Ʊ�NO����֤�仹ԭ�ԡ�������ڲ���C�Ƶ�NO��ȱ�ٵ�һ����Ҫ�����ǣ���ֹˮ�У��Ž�һ��������������֤NO�Ļ�ԭ�ԡ�
�ں��׳��ȼ�յIJ���ΪP2O5��
P2O5+6NaOH![]() 2Na3PO4+3H2O�����ӷ���ʽΪ��
2Na3PO4+3H2O�����ӷ���ʽΪ��
P2O5+6OH-![]() 2
2![]() +3H2O
+3H2O
�����ձ��е���ϡHNO3����������Ϊ��ͭƬ�����ܽ⣬����ɫ���ݲ�������Һ�����ɫ����Ӧ�����ӷ���ʽΪ��
3Cu+2![]() +8H+
+8H+![]() 3Cu2++2NO��+4H2O
3Cu2++2NO��+4H2O
(4)���¶���T1���ߵ�T2����������ʵ������Ӳ���Ϊ��ɫ��˵��NO2�����˷ֽⷴӦ��������ɫ���壬��������غ㶨�ɿ�֪��Ӧ����ʽΪ��
2NO2(g)![]() 2NO(g)+O2(g)��
2NO(g)+O2(g)��
�ڸ��ݷ�Ӧ�����������غ㣬��T2��T3ʱ���������Է�������Ϊ��![]() =30.7 g��mol-1������Է�������Ϊ30.7��
=30.7 g��mol-1������Է�������Ϊ30.7��



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 X��Y��Z��WΪ������ͬ�������ķ��ӻ����ӣ�����ԭ������С��10��Ԫ����ɣ�X��5��ԭ�Ӻˣ�ͨ��״���£�WΪ��ɫҺ�壮��֪��X+Y
X��Y��Z��WΪ������ͬ�������ķ��ӻ����ӣ�����ԭ������С��10��Ԫ����ɣ�X��5��ԭ�Ӻˣ�ͨ��״���£�WΪ��ɫҺ�壮��֪��X+Y
| ||
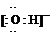
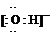
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
 ��2008?������X��Y��Z��WΪ������ͬ�������ķ��ӻ����ӣ�����ԭ������С��10��Ԫ����ɣ�X��5��ԭ�Ӻˣ�ͨ��״���£�WΪ��ɫҺ�壮
��2008?������X��Y��Z��WΪ������ͬ�������ķ��ӻ����ӣ�����ԭ������С��10��Ԫ����ɣ�X��5��ԭ�Ӻˣ�ͨ��״���£�WΪ��ɫҺ�壮| �� |


�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
X��Y��Z��WΪ������ͬ�������ķ��ӻ����ӣ�����ԭ������С��10��Ԫ����ɣ�X��5��ԭ�Ӻˡ�ͨ��״���£�WΪ��ɫҺ�塣
��֪:X+Y![]() Z+W
Z+W
(1)Y�ĵ���ʽ��_________________________��
(2)Һ̬Z��W�ĵ������ƣ����ɵ������������ͬ���������ӣ�Һ̬Z�ĵ��뷽��ʽ��_________________________________��
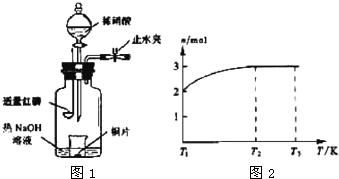
 (3)��ͼʾװ���Ʊ�NO����֤�仹ԭ�ԡ���������Ҫ������
(3)��ͼʾװ���Ʊ�NO����֤�仹ԭ�ԡ���������Ҫ������
a.����ƿ��ע��������NaOH��Һ����ʢ��ͭƬ��С�ձ�����ƿ�С�
b.�ر�ֹˮ�У���ȼ���ף�����ƿ�У����ý�����
c.�����׳��ȼ�գ�һ��ʱ����Һ©�����������ձ��е�������ϡ���ᡣ
�ٲ���c��ȱ�ٵ�һ����Ҫ������_______________________________________��
�ں��׳��ȼ�յIJ�����NaOH��Һ��Ӧ�����ӷ���ʽ�� ____________________________________________________________________��
�۲���c����ϡ������ձ��е�������______________________________________
______________________________________________________________________��
��Ӧ�����ӷ���ʽ��____________________________________________________��
 (4)һ���¶��£���1 mol N2O4�����ܱ������У�����ѹǿ���䣬�����¶���T1�Ĺ����У���������ɫ��Ϊ����ɫ���¶���T1�������ߵ�T2�Ĺ����У�������Ϊ��ɫ��������T2,����ѹǿ��������Ϊ����ɫ����������ʵ���n���¶�T�仯�Ĺ�ϵ��ͼ��ʾ��
(4)һ���¶��£���1 mol N2O4�����ܱ������У�����ѹǿ���䣬�����¶���T1�Ĺ����У���������ɫ��Ϊ����ɫ���¶���T1�������ߵ�T2�Ĺ����У�������Ϊ��ɫ��������T2,����ѹǿ��������Ϊ����ɫ����������ʵ���n���¶�T�仯�Ĺ�ϵ��ͼ��ʾ��
���¶���T1-T2֮�䣬��Ӧ�Ļ�ѧ����ʽ��_________________________��
���¶���T2-T3֮�䣬�����ƽ����Է��������ǣ�����1λС����______________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2008��߿��������ۻ�ѧ ���ͣ�ʵ����
(17��)X��Y��Z��WΪ������ͬ�������ķ��ӻ����ӣ�����ԭ������С��10��Ԫ����ɣ�X��5��ԭ�Ӻˡ�ͨ��״���£�WΪ��ɫҺ�塣
��֪:X+Y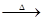 Z+W
Z+W
(1)Y�ĵ���ʽ��_________________________��
(2)Һ̬Z��W�ĵ������ƣ����ɵ������������ͬ���������ӣ�Һ̬Z�ĵ��뷽��ʽ��_________________________________��
(3)��ͼʾװ���Ʊ�NO����֤�仹ԭ�ԡ���������Ҫ������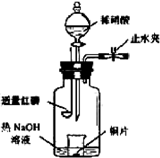
a.����ƿ��ע��������NaOH��Һ����ʢ��ͭƬ��С�ձ�����ƿ�С�
b.�ر�ֹˮ�У���ȼ���ף�����ƿ�У����ý�����
c.�����׳��ȼ�գ�һ��ʱ����Һ©�����������ձ��е�������ϡ���ᡣ
�ٲ���c��ȱ�ٵ�һ����Ҫ������_______________________________________��
�ں��׳��ȼ�յIJ�����NaOH��Һ��Ӧ�����ӷ���ʽ�� ____________________________________________________________________��
�۲���c����ϡ������ձ��е�������______________________________________
______________________________________________________________________��
��Ӧ�����ӷ���ʽ��____________________________________________________��
(4)һ���¶��£���1 mol N2O4�����ܱ������У�����ѹǿ���䣬�����¶���T1�Ĺ����У���������ɫ��Ϊ����ɫ���¶���T1�������ߵ�T2�Ĺ����У�������Ϊ��ɫ��������T2,����ѹǿ��������Ϊ����ɫ����������ʵ���n���¶�T�仯�Ĺ�ϵ��ͼ��ʾ��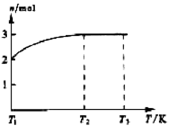
���¶���T1-T2֮�䣬��Ӧ�Ļ�ѧ����ʽ��_________________________��
���¶���T2-T3֮�䣬�����ƽ����Է��������ǣ�����1λС����______________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011-2012ѧ�����ʦ���и������Ĵ��¿���ѧ�Ծ� ���ͣ������
��12�֣�X��Y��Z��WΪ������ͬ�������ķ��ӻ����ӣ�����ԭ������С��10��Ԫ����ɣ�X��5��ԭ�Ӻˡ�ͨ��״���£�WΪ��ɫҺ�塣
��֪��X+Y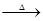 Z+W
Z+W
��1��Z�Ŀռ乹��Ϊ ��
��2��Һ̬Z��W�ĵ������ƣ����ɵ������������ͬ���������ӣ�Һ̬Z�ĵ��뷽��ʽ�� ��
��3��1mol��̬Z��O2��Ӧ����Һ̬W��һ��������Ԫ����ɵ��������ʣ��ų�������ΪQkJ��д���÷�Ӧ���Ȼ�ѧ����ʽ�� ��
��4��һ���¶��£���1mol N2O4�����ܱ������У�����ѹǿ���䣬�����¶���T1�Ĺ����У���������ɫ��Ϊ����ɫ���¶���T1�������ߵ�T2�Ĺ����У�������Ϊ��ɫ��������T2������ѹǿ��������Ϊ����ɫ����������ʵ���n���¶�T�仯�Ĺ�ϵ��ͼ��ʾ��
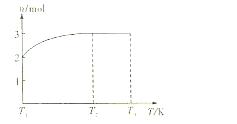
���¶���T1��T2֮�䣬��Ӧ�Ļ�ѧ����ʽ�ǣ� ��
���¶���T2��T3֮�䣬�����ƽ����Է��������ǣ�����1λС���� ��
������ʵ�����õ�ƽ��������ͨ��������ˮ�У���ʹ���屻��ȫ����������Ӧͬʱͨ���״���µĿ��� L������������Ϊ��N2��O2�������4��1��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com