
��1����һ���ݴӺ�������������Ĺ������¶ȱ��ֲ��䣬���ڴ˹����й��������е����壬
����˵����ȷ���� �� ������дѡ��ǰ����ĸ��
��A��������Ӽ������������ ��B��������ӵ�ƽ����������
��C��������ӵ�ƽ�����ܼ�С ��D��������ɵ�ϵͳ��������
��2�����������ڵ�������Ϊ�������壬���ݴӺ�������������Ĺ����У����������0.6J�Ĺ�����˹����е����� �� ������ա��ų������������� �� J�����ݵ��������¶������Ĺ����У��ֶ��������0.1J�Ĺ���ͬʱ������0.3J����������˹����У��������������������� �� J
��3����֪������������ܶ�Ϊ1.29kg/![]() ��ƽ��Ħ������Ϊ0.29kg/mol�������ӵ�����
��ƽ��Ħ������Ϊ0.29kg/mol�������ӵ�����
![]() ��ȡ������ӵ�ƽ��ֱ��Ϊ
��ȡ������ӵ�ƽ��ֱ��Ϊ![]() ���������ڵ���������ȫ��ΪҺ�壬�����Һ�������ԭ����������ı�ֵ�������������Ϊ��Ч���֣�
���������ڵ���������ȫ��ΪҺ�壬�����Һ�������ԭ����������ı�ֵ�������������Ϊ��Ч���֣�
 ��ĩ100�ִ��غ�������ϵ�д�
��ĩ100�ִ��غ�������ϵ�д� Сѧ�������Ծ�ϵ�д�
Сѧ�������Ծ�ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ���������� ��Դ�� ���ͣ��Ķ�����
| 4 | 3 |
�鿴�𰸺ͽ���>>
��Ŀ���������� ��Դ�� ���ͣ�
��1����һ���ݴӺ�������������Ĺ������¶ȱ��ֲ��䣬���ڴ˹����й��������е����壬����˵����ȷ���� ������дѡ��ǰ����ĸ��
��A��������Ӽ������������ ��B��������ӵ�ƽ����������
��C��������ӵ�ƽ�����ܼ�С ��D��������ɵ�ϵͳ��������
��2�����������ڵ�������Ϊ�������壬���ݴӺ�������������Ĺ����У����������0.6J�Ĺ�����˹����е����� ������ա��ų������������� J�����ݵ��������¶������Ĺ����У��ֶ��������0.1J�Ĺ���ͬʱ������0.3J����������˹����У��������������������� J��
��3����֪������������ܶ�Ϊ1.29kg/![]() ��ƽ��Ħ������Ϊ0.29kg/mol�������ӵ�����
��ƽ��Ħ������Ϊ0.29kg/mol�������ӵ�����![]() ��ȡ������ӵ�ƽ��ֱ��Ϊ
��ȡ������ӵ�ƽ��ֱ��Ϊ![]() ���������ڵ���������ȫ��ΪҺ�壬�����Һ�������ԭ����������ı�ֵ�����������һλ��Ч���֣���
���������ڵ���������ȫ��ΪҺ�壬�����Һ�������ԭ����������ı�ֵ�����������һλ��Ч���֣���
�鿴�𰸺ͽ���>>
��Ŀ���������� ��Դ�����˽̰��������ѡ��3-3���Ӷ����ۡ�����ר����ϰ���������� ���ͣ�������
��09�����վ�������12.A����ѡ��ģ��3��3����12�֣���1����һ���ݴӺ�������������Ĺ������¶ȱ��ֲ��䣬���ڴ˹����й��������е����壬����˵����ȷ���ǣ� �� ������дѡ��ǰ����ĸ��
��A��������Ӽ������������ ��B��������ӵ�ƽ����������
��C��������ӵ�ƽ�����ܼ�С ��D��������ɵ�ϵͳ��������
��2�����������ڵ�������Ϊ�������壬���ݴӺ�������������Ĺ����У����������0.6J�Ĺ�����˹����е����� ������ա��ų������������� J�����ݵ��������¶������Ĺ����У��ֶ��������0.1J�Ĺ���ͬʱ������0.3J����������˹����У��������������������� J��
��3����֪������������ܶ�Ϊ1.29kg/ ��ƽ��Ħ������Ϊ0.29kg/mol�������ӵ�����
��ƽ��Ħ������Ϊ0.29kg/mol�������ӵ����� ��ȡ������ӵ�ƽ��ֱ��Ϊ
��ȡ������ӵ�ƽ��ֱ��Ϊ ���������ڵ���������ȫ��ΪҺ�壬�����Һ�������ԭ����������ı�ֵ�����������һλ��Ч���֣���
���������ڵ���������ȫ��ΪҺ�壬�����Һ�������ԭ����������ı�ֵ�����������һλ��Ч���֣���
�鿴�𰸺ͽ���>>
��Ŀ���������� ��Դ�����˽̰��������ѡ��3-3���Ӷ����ۡ�����ר����ϰ�������棩 ���ͣ�������
��09�����վ�������12.A����ѡ��ģ��3��3����12�֣���1����һ���ݴӺ�������������Ĺ������¶ȱ��ֲ��䣬���ڴ˹����й��������е����壬����˵����ȷ���ǣ� �� ������дѡ��ǰ����ĸ��
��A��������Ӽ������������ ��B��������ӵ�ƽ����������
��C��������ӵ�ƽ�����ܼ�С ��D��������ɵ�ϵͳ��������
��2�����������ڵ�������Ϊ�������壬���ݴӺ�������������Ĺ����У����������0.6J�Ĺ�����˹����е����� ������ա��ų������������� J�����ݵ��������¶������Ĺ����У��ֶ��������0.1J�Ĺ���ͬʱ������0.3J����������˹����У��������������������� J��
��3����֪������������ܶ�Ϊ1.29kg/ ��ƽ��Ħ������Ϊ0.29kg/mol�������ӵ�����
��ƽ��Ħ������Ϊ0.29kg/mol�������ӵ����� ��ȡ������ӵ�ƽ��ֱ��Ϊ
��ȡ������ӵ�ƽ��ֱ��Ϊ ���������ڵ���������ȫ��ΪҺ�壬�����Һ�������ԭ����������ı�ֵ�����������һλ��Ч���֣���
���������ڵ���������ȫ��ΪҺ�壬�����Һ�������ԭ����������ı�ֵ�����������һλ��Ч���֣���
�鿴�𰸺ͽ���>>
��Ŀ���������� ��Դ��2010���������ÿ�ܾ�����������ѧ ���ͣ������
��1����һ���ݴӺ�������������Ĺ������¶ȱ��ֲ��䣬���ڴ˹����й��������е����壬����˵����ȷ���� ������дѡ��ǰ����ĸ��
��A��������Ӽ������������ ��B��������ӵ�ƽ����������
��C��������ӵ�ƽ�����ܼ�С ��D��������ɵ�ϵͳ��������
��2�����������ڵ�������Ϊ�������壬���ݴӺ�������������Ĺ����У����������0.6J�Ĺ�����˹����е����� ������ա��ų������������� J�����ݵ��������¶������Ĺ����У��ֶ��������0.1J�Ĺ���ͬʱ������0.3J����������˹����У��������������������� J��
��3����֪������������ܶ�Ϊ1.29kg/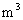 ��ƽ��Ħ������Ϊ0.29kg/mol�������ӵ�����
��ƽ��Ħ������Ϊ0.29kg/mol�������ӵ�����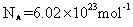 ��ȡ������ӵ�ƽ��ֱ��Ϊ
��ȡ������ӵ�ƽ��ֱ��Ϊ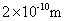 ���������ڵ���������ȫ��ΪҺ�壬�����Һ�������ԭ����������ı�ֵ�����������һλ��Ч���֣���
���������ڵ���������ȫ��ΪҺ�壬�����Һ�������ԭ����������ı�ֵ�����������һλ��Ч���֣���
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com