
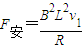 ������ţ�ٵڶ���������ٶȣ�
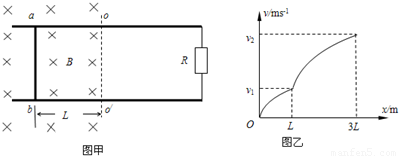
������ţ�ٵڶ���������ٶȣ� �⣺��1��ab����λ��L��3L�Ĺ����У��ɶ��ܶ���
�⣺��1��ab����λ��L��3L�Ĺ����У��ɶ��ܶ���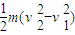
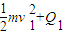
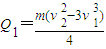
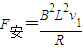
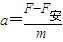
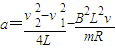
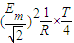
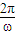
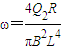
 ��ab�����뿪�ų�ǰ˲��ļ��ٶ�Ϊ
��ab�����뿪�ų�ǰ˲��ļ��ٶ�Ϊ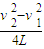 -
-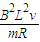 ��
��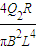 ��
��


| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ���������� ��Դ�� ���ͣ��Ķ�����
| 4 | 3 |
�鿴�𰸺ͽ���>>
��Ŀ���������� ��Դ�� ���ͣ��Ķ�����
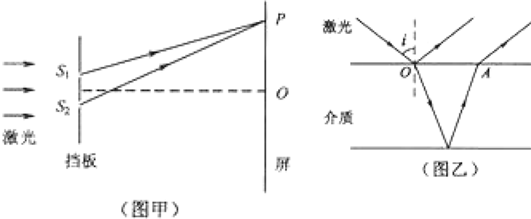
�鿴�𰸺ͽ���>>
��Ŀ���������� ��Դ�� ���ͣ�
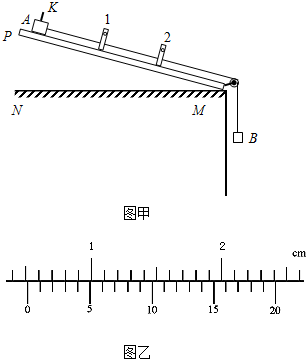
 ��2011?����ģ�⣩�����ʱ��һ��������ʱ��Ҳ��һ���о������˶�����ij�����ʱ������ÿ������Ŷ��ɹⷢ��ͽ���װ����ɣ���������ӹ�����м�ͨ��ʱ����֮�����ļ�ʱ���Ϳ�����ʾ����ĵ���ʱ�䣮��ͼ����ʾ��ͼ����NM��ˮƽ���桢PM��һ�˴��л��ֵij�ľ�壬1��2�ǹ̶���ľ�������Ϊl����������ţ���֮���ӵļ�ʱ��û�л�����������A�Ϲ̶������ڵ����խƬK���û���A������B��ǣ���´�ľ��Ķ��˻��£���ʱ���ֱ���ʾխƬKͨ�������1�����2�ĵ���ʱ��ֱ�Ϊt1=2.50��10-2s��t2=1.25��10-2s��
��2011?����ģ�⣩�����ʱ��һ��������ʱ��Ҳ��һ���о������˶�����ij�����ʱ������ÿ������Ŷ��ɹⷢ��ͽ���װ����ɣ���������ӹ�����м�ͨ��ʱ����֮�����ļ�ʱ���Ϳ�����ʾ����ĵ���ʱ�䣮��ͼ����ʾ��ͼ����NM��ˮƽ���桢PM��һ�˴��л��ֵij�ľ�壬1��2�ǹ̶���ľ�������Ϊl����������ţ���֮���ӵļ�ʱ��û�л�����������A�Ϲ̶������ڵ����խƬK���û���A������B��ǣ���´�ľ��Ķ��˻��£���ʱ���ֱ���ʾխƬKͨ�������1�����2�ĵ���ʱ��ֱ�Ϊt1=2.50��10-2s��t2=1.25��10-2s���鿴�𰸺ͽ���>>
��Ŀ���������� ��Դ������ģ�� ���ͣ��ʴ���
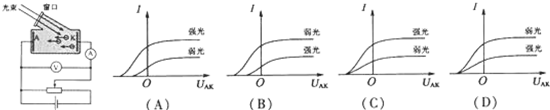
�鿴�𰸺ͽ���>>
��Ŀ���������� ��Դ��2011�꽭��ʡ�߿�����ģ���Ծ��������������棩 ���ͣ������
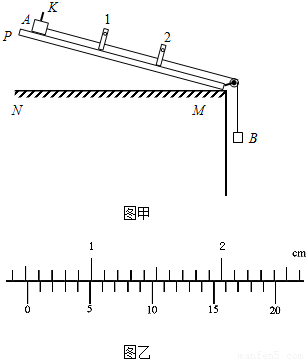
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com