
题目列表(包括答案和解析)
(7分)(1)已知:H—H键能为436kJ/mol,N≡N键能为945kJ/mol,N—H键能为391kJ/mol。根据键能计算工业合成氨的反应是___________ 反应(填“吸热”或“放热”),消耗1mol N2合成氨反应的 =______________。
=______________。
(2) 现根据下列3个热化学反应方程式:
①Fe2O3(s)+3CO(g) =2Fe(s)+3CO2(g) △H=―24.8kJ/mol
②3Fe2O3(s)+ CO(g) =2Fe3O4(s)+ CO2(g) △H=―47.2kJ/mol
③Fe3O4(s)+CO(g) =3FeO(s)+CO2(g) △H=+640.5kJ/mol
则CO气体还原FeO固体得到Fe固体和CO2气体的热化学反应方程式为 。
(7分)(1)已知:H—H键能为436kJ/mol,N≡N键能为945kJ/mol,N—H键能为391kJ/mol。根据键能计算工业合成氨的反应是___________ 反应(填“吸热”或“放热”),消耗1mol N2合成氨反应的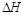 =______________。
=______________。
(2) 现根据下列3个热化学反应方程式:
①Fe2O3(s)+3CO(g) =2Fe(s)+3CO2(g) △H=―24.8kJ/mol
②3Fe2O3(s)+ CO(g) =2Fe3O4(s)+ CO2(g) △H=―47.2kJ/mol
③Fe3O4(s)+CO(g) =3FeO(s)+CO2(g) △H=+640.5kJ/mol
则CO气体还原FeO固体得到Fe固体和CO2气体的热化学反应方程式为 。
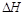 =______________。
=______________。 盖斯定律在生产和科学研究中有很重要的意义。有些反应的反应热虽然无法直接测得,但可通过间接的方法测定。现根据下列3个热化学反应方程式:
Fe2O3(s)+3CO(g)==2Fe(s)+3CO2(g) △H= ―24.8kJ/mol
3Fe2O3(s)+CO(g)==2Fe3O4(s)+ CO2(g) △H= ―47.2kJ/mol
Fe3O4(s)+CO(g)==3FeO(s)+CO2(g) △H= +640.5kJ/mol
写出CO气体还原FeO固体得到Fe固体和CO2气体的热化学反应方程式:
_________________
(4分)盖斯定律在生产和科学研究中有很重要的意义。有些反应的反应热虽然无法直接测得,但可通过间接的方法测定。现根据下列3个热化学反应方程式:
Fe2O3(s)+3CO(g)= 2Fe(s)+3CO2(g) △H= ―24.8kJ/mol
3Fe2O3(s)+ CO(g)=2Fe3O4(s)+ CO2(g) △H= ―47.2kJ/mol
Fe3O4(s)+CO(g)=3FeO(s)+CO2(g) △H= +640.5kJ/mol
写出CO气体还原FeO固体得到Fe固体和CO2气体的热化学反应方程式:
________________
湖北省互联网违法和不良信息举报平台 | 网上有害信息举报专区 | 电信诈骗举报专区 | 涉历史虚无主义有害信息举报专区 | 涉企侵权举报专区
违法和不良信息举报电话:027-86699610 举报邮箱:58377363@163.com