
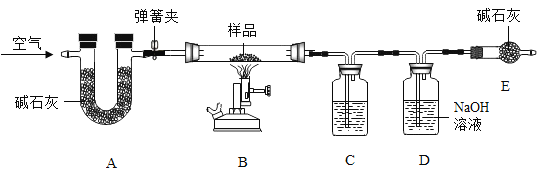
����Ŀ����ˮƿ�þú�ƿ���ڱڸ���һ��ˮ������ɷ�Ϊ̼��ơ�������þ������ˮ�� ʵ��������һƿˮ����Ʒ��Ϊ�ⶨ���и��ɷֵ�����������ij��ѧ��ȤС���ͬѧ����ͼ��ʾװ�ý���ʵ�飨��װ�����������ã�A��C��D��E ����װҩƷ����������֪��ʯ���������ƺ��������ƵĻ������������£�������þ�ֽ⣬��Ӧ����ʽΪ��Mg��OH��2![]() MgO + H2 O��
MgO + H2 O��
����ʵ�鲽�����£�
��.������Ϊ m ����Ʒװ��װ�� B �IJ������У���ͼ���Ӻ�װ�ã��رյ��ɼУ�����Ʒ���ȣ�
��.����Ʒ��ȫ��Ӧ���ȴ��ɼ�ͨ���������Ϩ��ƾ��ƣ�ֱ����������ȴ��
��.ʵ����ϣ����װ�� C��D �е�Һ�������ֱ�������m1��m2��
��.����ʵ���������ݼ������Ʒ��̼��ơ�������þ��ˮ������������ ��ش��������⣺
��1��װ�� C ����װҩƷΪ____________��
��2��װ�� E ������Ϊ____________��
��3��װ�� D �з�Ӧ�ķ���ʽΪ____________��
��4����ˮ����Ʒ��̼��Ƶ�������������ʽΪ____________��
��5��ʵ�鷴˼��ʵ�鲽�費�䣬��û��װ�� A���ᵼ��̼��ƵIJⶨ���____________������ƫ��������ƫС���������жϣ���ͬ��������û��װ�� E���ᵼ��������þ�IJ�ý��____________��
���𰸡�Ũ���� ���տ����еĶ�����̼��ˮ������ֹ����װ��D�� CO2 + 2NaOH = Na2CO3 + H2O ![]() ƫ�� ƫС
ƫ�� ƫС
��������
װ��A�еļ�ʯ����Ҫ��Ϊ���ų������е�ˮ�����Ͷ�����̼�ĸ��ţ���װ��B�м�����Ʒ��������������̼��ˮ������ʵ��Ŀ����Ϊ�˼������Ʒ��̼��ơ�������þ��ˮ����������������������Ҫ������ɵ�ˮ�Ͷ�����̼��������
��1��װ��D�е�����������Һ��Ҫ��Ϊ�˲�����ɵĶ�����̼������������װ��C�е���ҺӦ�ÿ��Բ�����ɵ�ˮ����������ӦΪŨ���ᣬ���Ũ���ᡣ
��2��װ��E�����տ����еĶ�����̼��ˮ������ֹ����װ��D�Բ���������Ӱ�죬������տ����еĶ�����̼��ˮ������ֹ����װ��D��
��3��װ��D�е�����������Һ�������ɵĶ�����̼����̼���ƺ�ˮ���仯ѧ����ʽΪCO2 + 2NaOH = Na2CO3 + H2O�����CO2 + 2NaOH = Na2CO3 + H2O��
��4��DҺ�����ӵ�����ʵ������̼��Ʒֽ����ɵĶ�����̼����������̼�������Ϊx��
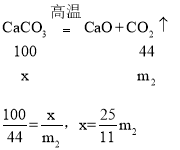
��ˮ����Ʒ��̼��Ƶ�������������ʽΪ��![]()
��5����û��װ�� A�������еĶ�����̼�ᱻD���գ�m2ƫ��̼�������ƫ�ᵼ��̼��ƵIJⶨ���ƫ����û��װ�� E�������е�ˮ����������̼����D��ͬ���ᵼ��m2ƫ��̼�������ƫ�ᵼ��̼��ƵIJⶨ���ƫ����������þ�IJ�ý��ƫС�����ƫ��ƫС��



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ר����ʾ���ճ���������״�����У���84������Һ����ʹ������

��1������Ч�ɷ� NaClO ����Ԫ�صĻ��ϼ�Ϊ______________��������������Ϊ_____________��д���ӷ��ţ���
��2����84������Һ������ԭ��Ϊ 2NaClO+CO2+X=Na2CO3+2HClO�����ɵ� HClO����������н�ǿ��ɱ�� ���á���ѧ����ʽ�� X �Ļ�ѧʽΪ_____________��
��3����ͼ�������г���ϴ����Ʒ�ĵ� pH�����ûή��ȥ��Ч����������Σ���������_____________������ţ���

A��������84������Һ B����ˮ����������
C��84������Һ�ͷ���ˮ D��84������Һ����������
��4��̽�����֣���¶�ڿ����У�������㣩����84������Һ������Ч����ʱ������ƶ��������ݴˣ�����Ϊ��84������ҺӦ________���档
��5����84������Һ��һ���Ĵ̼����븯ʴ�ԣ�����ϡ���Ժ����ʹ�á��� 50g �� NaClO 5%����84������Һϡ���� 1%������ԭ��Һ�м���ˮ������Ϊ_____________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��A��G��ʾ���л�ѧ���������ʣ�����֮���ת����ϵ��ͼ��ʾ����������������ȥ��������A��B��C�о�����ͬһ��Ԫ�أ�D���������壬G����Ҫ��ζƷ����ش��������⣺
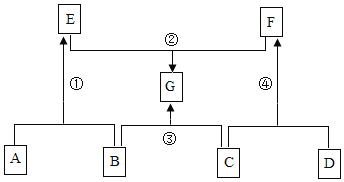
��1��G�Ļ�ѧʽΪ_____��
��2����Ӧ���Ļ�����Ӧ����Ϊ��_____��
��3����Ӧ���Ļ�ѧ����ʽΪ��_____��
��4��F��һ����;�ǣ�_____��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����1����ͼ�Ǽס������ֹ������ʵ��ܽ�����ߣ���������������������գ�
��20��ʱ��_______���ʵ��ܽ��Ϊ40g��
��30��ʱ���Ѽס��������ʸ�100g�ֱ����Ʊ�����Һ����Ҫ�ܼ������϶����_____��
��30��ʱ������ͬ�����ļ��ҵı�����Һ�ֱ���ȴ��0�棬����������϶����____��
�ܵ����к�������������ʱ���ɲ���______�������������������������ᾧ�ķ����ᴿ������.
��2�������б������Ԫ������������п��������ȣ���Ȼ�����٣����Խ���������Ҫ�������ṩ������Ԫ�ص������Ϣ��������������ش��������⡣
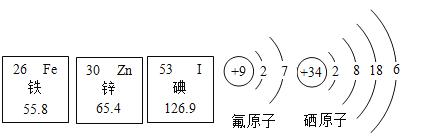
��������Ԫ����ȱ��___����ɼ�״���״�.
��пԭ�Ӻ��������Ϊ____.
����ԭ���ڻ�ѧ��Ӧ�����õ�2�����ӣ��γ�_____������������������������.
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
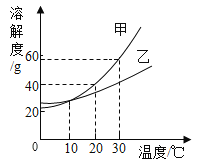
����Ŀ����ͼΪ���� M�������ᾧˮ�����ܽ�����ߣ�a��b����ֱ��ʾ M ���ʵ���Һ�������й�˵����ȷ�ĸ�����
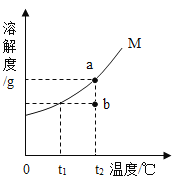
��ͬѧ��t2ʱ��a�DZ�����Һ��b�Dz�������Һ��������Һ�������
��ͬѧ����������Һ�¶Ƚ���t1ʱ��a��b���о�������
��ͬѧ����a![]() b���Ƚ�a���µ�t1�����˺���Һ���µ�t2
b���Ƚ�a���µ�t1�����˺���Һ���µ�t2
��ͬѧ����b![]() a�������¶Ȳ��䣬��b�м���M���պñ���
a�������¶Ȳ��䣬��b�м���M���պñ���
A.1B.2C.3D.4
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����ѧ�����ǹ���ͨ�����ԣ����û�ѧ������գ�
��1����Ԫ��_______��
��2����ԭ��_____��
��3��2��������������_______��
��4��þ����________��
��5�����������__________��
��6�����H2O2����Ԫ�صĻ��ϼ�______��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�������ʻ�ϡ���Ӧ�ȹ����У���������1+1��2������Ȥ����ͨ������£�����ѡ�������1+1 <2������
A.1L�ƾ���1Lˮ��Ϻ���Һ�����
B.1L������IL������Ӧ����������
C.1g����������Һ��1gϡ���ᷴӦ����Һ������
D.1g����������Һ��1gϡ���ᷴӦ����Һ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ѧϰС��ⶨij��ҵ��ˮ(����H2SO4��HNO3������������)��H2SO4�ĺ�����ȡ100g��ˮ���ձ��У�����100g BaCl2��Һ��ǡ����ȫ��Ӧ�������˵õ�176.7g��Һ��
��1����ַ�Ӧ�����ɳ���������Ϊ______g��
��2���ù�ҵ��ˮ���������������Ϊ����?(д���������)______��
��3��Ϊ���ҵ��ˮ��Ⱦ�������ŷ�ǰӦ�Է�ˮ�����кʹ�����������������_______��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�������dz��л�ѧ��һЩ����ʵ�飬�����ʵ�����ݻش��������⡣
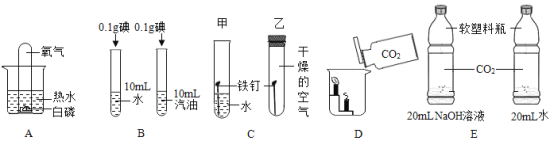
(1)Aʵ���У���ʢ���������Թܵ����ڱ���ˮ��û�İ����Ϸ������Թ۲쵽��������____��
(2)Bʵ���Ŀ����______��
(3)Cʵ���У����Թܵ��������⣬���Թܵ����������⣬˵������������_________�йء�
(4)Dʵ���������_______��
(5)Eʵ���У�������ƿ�������ʢ������������Һ��ƿ�ӱ�ʢ��ˮ��ƿ�����Ը��ݴ�������Եó��Ľ�����______��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com