
����Ŀ����������ʵ���ó�����Ӧ������ȷ����
ʵ����ʵ | ���� | |
A | ����ͬ�¶��£���1 mL0.2 mol/LNaOH��Һ�е���2��0.1 mol/LMgCl2��Һ��������ɫ�������ٵμ�2��0.1 mol/LFeCl3��Һ�������ɺ��ɫ���� | �ܽ�ȣ�Mg(OH)2>Fe(OH)3 |
B | ij������ʹʪ�����ɫʯ����ֽ��� | ������ˮ��Һһ���Լ��� |
C | ͬ��ͬѹ�£������pH=3��HA��HB������ֱ���������п��Ӧ����ˮ���ռ����壬HA�ų����������ҷ�Ӧ���ʿ� | HB�����Ա�HAǿ |
D | SiO2����������ᷴӦ������Ӧ | SiO2������������ |
A.AB.BC.CD.D
���𰸡�C
��������
A. ������ӦMgCl2+2NaOH=Mg(OH)2��+2NaCl������NaOH����������ٵ���FeCl3��Һ���ᷢ����Ӧ��FeCl3+3NaOH=Fe(OH)3��+3NaCl�����ܱȽ�Mg(OH)2��Fe(OH)3�ܽ�ȴ�С��A����
B. ij������ʹʪ�����ɫʯ����ֽ��죬�������Ϊ�������壬��ˮ��Һ�����ԣ�B����
C. HA�ų����������ҷ�Ӧ���ʿ죬HAŨ�ȱ�HB���ڷ�Ӧ������HA��Һ��c(H+)�Ƚϴ�֤��HA��Һ�д��ڵ���ƽ��HA![]() H++A-��HA�����ᣬ������HB>HA��C��ȷ��
H++A-��HA�����ᣬ������HB>HA��C��ȷ��
D. SiO2������ᷴӦ����SiF4��H2O��SiF4�����Σ����SiO2�������������D����
�ʺ���ѡ����C��


 ��У���˿��ֿ���ϵ�д�
��У���˿��ֿ���ϵ�д� �ƸԴ��ž�ϵ�д�
�ƸԴ��ž�ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����ͭ��������������к���Cu��Au(��)��PbSO4�����ʣ�ʪ����������������ۺ����õĹ���������ͼ��ʾ��
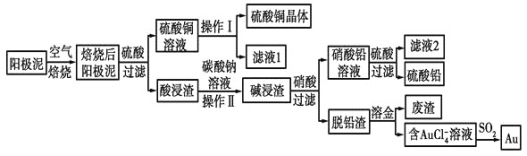
(1)��⾫����ͭ����Ǧ�Ĵ�ͭʱ�����ҺӦ����________��Һ�����Һ�����ʱ�����ĵ缫��ӦʽΪ___________________________��Cu-2e-=Cu2+��
(2)��ɲ���������Ҫ�����У�__________________�����ˣ�ϴ�ӣ����
(3)д����SO2��ԭAuCl4-�����ӷ�Ӧ����ʽ____________________________��
(4)Ϊ�˼��ٷ�Һ�ŷš��������������Դ����ҵ�Ͻ���Һ1��������ͭ��Һ����ѭ����������ָ������ͼ����һ�����Ƶ�����________________________��
(5)�����ӷ���ʽ��ʾ����̼������Һ�����ã�___________________________��[��֪298Kʱ��Ksp(PbCO3)=1.46��10-13��Ksp(PbSO4)=1.82��10-8]������Һ��c(SO42-)=0.2mol/Lʱ��c(CO32-)=________mol/L��![]() �������2λ��Ч����
�������2λ��Ч����![]()
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��������(Mo)�����ڹ�ҵ�����µ������ѱ����������Ϊս�Խ������ҹ����ⴢ��������ڶ����ش���������
��1��Mo��Cr��ͬ��Ԫ�أ�����λ���������ڣ�д����̬Moԭ�ӵļ۵��ӵĹ������ʽΪ_____________��
��2������(MoS2)���������豸��������ȹ����ϩ(��C60)�������ƣ�����H2S��(NH4)2MoO4��Һ�������⡣H2S����VSEPRģ��Ϊ____________��(NH4)2MoO4������Ԫ�صĵ�һ�������ɴ�С��˳��Ϊ________________��MoS2�������Ӿ���������ʯī�IJ�״�ṹ����������������ܣ���ԭ����___________________________��
��3��̼����������������ԭ������ԭ��ΪMoS2+4H2+2Na2CO3![]() Mo+2CO+4H2O+2Na2S�����ӻ������еĴ��������÷���
Mo+2CO+4H2O+2Na2S�����ӻ������еĴ��������÷���![]() ��ʾ������m���������γɵĴ�����ԭ������n���������γɵĴ��������������籽�����еĴ������ɱ�ʾΪ
��ʾ������m���������γɵĴ�����ԭ������n���������γɵĴ��������������籽�����еĴ������ɱ�ʾΪ![]() ������̼������CO32-�����еĴ�����Ӧ��ʾΪ________��
������̼������CO32-�����еĴ�����Ӧ��ʾΪ________��
��4�����һ������ﻯѧʽΪ��Na3[Mo(CN)8]8H2O�����г����ۼ�����λ��������ڵ���������_____________����������������������������Ŀ֮��Ϊ_________��
��5�������⾧���е�ԭ�Ӷѻ���ʽ��ͼ��ʾ�����ֶѻ���ʽΪ_________�ѻ�������������ܶ�Ϊ��g��cm��3����ԭ�Ӱ뾶Ϊr pm��NA��ʾ�����ӵ�������ֵ��M��ʾ������ԭ�����������⾧����ԭ�ӵĿռ�������Ϊ__________________(�ú�������r��NA��M�Ĵ���ʽ��ʾ)��

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���������������ˮ����Ѫѹ��ҩ����м���G��һ�ֺϳ�·�����£�
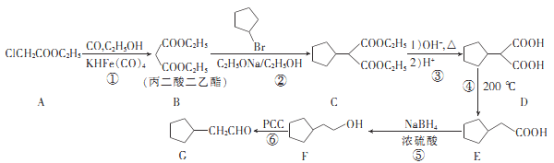
�ش���������:
(1)A�Ļ�ѧ������__________��B�к��й����ŵ�����Ϊ___________��
(2)��Ӧ�ڵķ�Ӧ������____________��
(3)G������Cu(OH)2��Ӧ�Ļ�ѧ����ʽΪ__________________��
(4)X��E��Ϊͬ���칹�壬X�к�����Ԫ̼������X����NaOH��Һ��Ӧ�������������X�Ľṹ��ʽΪ___________________��
(5)�����1,3-�������ͱ�����������Ʊ�![]() �ĺϳ�·��(�����Լ���ѡ)��_____________
�ĺϳ�·��(�����Լ���ѡ)��_____________
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���������ϡ����ǽ������ϡ��л��߷��Ӳ���������ʹ�õ�������������ϣ������Ը��Ե��ص����������������Ҫ��
(1)���������У���һ������Ͻ��ܹ���������γɽ������������һ�������·ֽ��ͷų�������������ԭ������_______�仯�������������ִ�����Ҫ�������ϣ�������ͨ��װ��һ����Ŀ���������õĽ������Է�ֹ��ʴ���ý��������ѡ��_______��ѡ����ͭ��������п��������Ǧ��������
(2)���ǽ��������У����ڵ��ӹ�ҵ�ĸߴ�̼��ơ��ߴ������������������£�

��ش��������⣺
���������̼�������Һ�з�Ӧ���������Ӧ����Ϊ_______��
��ʵ���ҳ�����_______�������г������룻
�������ߴ�̼���ʱ��ѡ����220����¸���������ѡ������������ԭ����_______��
�ܸߴ����������������У�������������ҪĿ����Ϊ�˷�ֹ______���û�ѧ����ʽ��ʾ����
(3)�л��߷��Ӳ���������������������ɽ��ⱻ�㷺�������һ������ĭ���ϣ���������������ʹ�ÿɼ���_______��Ⱦ��20����30���������������ϸ˿�����˿�����������磬�����пɲ���______�������������Ͳ�˿��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��NM3�Ǵ����ٴ�����ε�С���ӿ���ҩ����ӽṹ��ͼ������˵����ȷ����
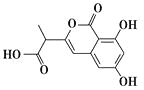
A.���л���ķ���ʽΪC12H12O6
B.1 mol���л��������Ժ�3 mol NaOH��Ӧ
C.���л����������ӳɡ�ȡ������ȥ�ȷ�Ӧ
D.���л��������ֻ����1������̼ԭ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���⻯����ҽ�Ƽ�ʳƷ��������Ҫ�����á�ʵ������NaOH�����ʵ��ˮ����(N2H4��H2O)Ϊԭ���Ʊ��⻯�ơ���֪��ˮ���¾��л�ԭ�ԡ��ش��������⣺
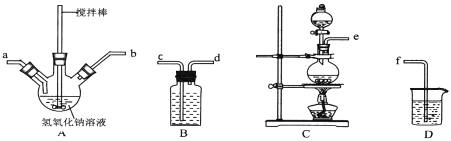
(1)ˮ���µ��Ʊ���Ӧԭ��Ϊ��CO(NH2)2(����)+NaClO+2NaOH= N2H4��H2O +NaCl+Na2CO3
����ȡ�������ƺ��������ƻ��Һ������˳��Ϊ__________(������������Сд��ĸ��ʾ)������ʵ���¶ȿ��Ʋ�������Ӧ��������ƿ��ClO-��ClO3-�����ʵ���֮��Ϊ5��1�����������������Ʒ�Ӧʱ������ԭ����Ԫ���뱻��������Ԫ�ص����ʵ���֮��Ϊ____��
���Ʊ�ˮ����ʱ��Ӧ��__________�ε�__________�У�����NaClO��Һ������������Һ�������ҵμ��ٶȲ��ܹ��졣
�����صĵ���ʽΪ__________________
(2)�⻯�Ƶ��Ʊ�������ˮ���»�ԭ����ȡ�⻯�ƹ��壬���Ʊ�������ͼ��ʾ��
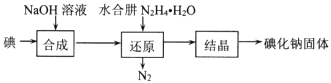
������ԭ�������У���Ҫ���ķ�Ӧ���������ɵĸ�����IO3-���ù��̵����ӷ���ʽΪ ___��
(3)�ⶨ��Ʒ��NaI������ʵ�鲽�����£�
a����ȡ10.00 g��Ʒ���ܽ⣬��500 mL����ƿ�ж��ݣ�
b����ȡ25.00 mL����Һ����ƿ�У�Ȼ�����������FeCl3��Һ����ַ�Ӧ���ټ���M��Һ��ָʾ����
c. ��0.2000 mol��L1�� Na2S2O3�� �� Һ �� �� �� �� ��(�� Ӧ �� �� ʽ2Na2S2O3+I2=Na2S4O6+2NaI)���ظ�ʵ���Σ�������ı���Һ�����Ϊ15.00 mL��
��MΪ____________(д����)��
�ڸ���Ʒ��NaI����������Ϊ_______________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ijһ������ķ���ʽΪAB2��A����A��Ԫ�أ�B����A��Ԫ�أ�A��B��ͬһ���ڣ����ǵĵ縺��ֵ�ֱ�Ϊ3.44��3.98����֪AB2���ӵļ���Ϊ103.3���������ƶϲ���ȷ���ǣ� ��
A.AB2���ӵĿռ乹��Ϊ��V����
B.A---B��Ϊ���Թ��ۼ���AB2����Ϊ�Ǽ��Է���
C.AB2��H2O��ȣ�AB2���۵㡢�е��H2O�ĵ�
D.AB2����������ԭ�ӣ����Ӽ䲻���γ��������H2O���Ӽ����γ����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
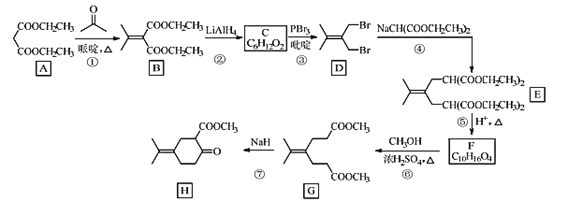
����Ŀ��������H��һ�����Ϻϳ��м��壬��ϳ�·����ͼ��
�ش��������⣺
(1)A�Ļ�ѧ������___��
(2)A��B�ķ�Ӧ����ʽ��___��
(3)C�Ľṹ��ʽ��___��
(4)�ܵķ�Ӧ������___��
(5)H�еĺ��������ŵ�������___��
(6)X��F��ͬ���칹�壬X���������ŵ������������F��ȫ��ͬ����˴Ź�������Ϊ����壬�������Ϊ6��1��1��д�����ַ�������������X�Ľṹ��ʽ___��
(7)����ɱ��״��� �Ʊ�
�Ʊ�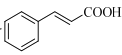 �ĺϳ�·��___�����Լ���ѡ����
�ĺϳ�·��___�����Լ���ѡ����
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com