
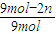 =0.6��n=1.8mol����
=0.6��n=1.8mol���� 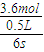 =1.2mol?L-1?s-1��
=1.2mol?L-1?s-1��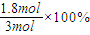 =60%��
=60%��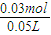 =0.6mol/L��
=0.6mol/L��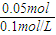 =0.5L=500ml��
=0.5L=500ml��

 Сѧ��ʱ��ҵȫͨ����ϵ�д�
Сѧ��ʱ��ҵȫͨ����ϵ�д� �����ÿ�ʱѵ��ϵ�д�
�����ÿ�ʱѵ��ϵ�д� ��Ԫȫ��������ϵ�д�
��Ԫȫ��������ϵ�д� �»ƸԱ����ܾ�ϵ�д�
�»ƸԱ����ܾ�ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 ��2014?����һģ���̶�������CO2������Ч��������Դ�������ٿ����е��������壮��ҵ�������о�����CO2�������״�ȼ�ϵķ������÷����Ļ�ѧ����ʽ�ǣ�
��2014?����һģ���̶�������CO2������Ч��������Դ�������ٿ����е��������壮��ҵ�������о�����CO2�������״�ȼ�ϵķ������÷����Ļ�ѧ����ʽ�ǣ��鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
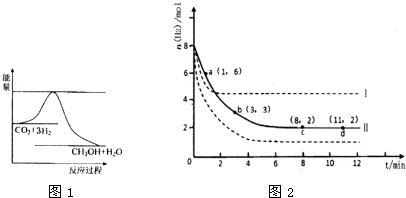
 ��2012?��������ģ��T��ʱ����6mol CO2��8mol H2����2L�ܱ������У�������ӦCO2��g��+3H2��g��?CH3OH��g��+H2O��g����������H2�����ʵ�����ʱ��仯��ͼ��ʵ����ʾ��ͼ�����߱�ʾ���ı�ijһ��Ӧ����ʱ��H2�����ʵ�����ʱ��ı仯������˵����ȷ���ǣ�������
��2012?��������ģ��T��ʱ����6mol CO2��8mol H2����2L�ܱ������У�������ӦCO2��g��+3H2��g��?CH3OH��g��+H2O��g����������H2�����ʵ�����ʱ��仯��ͼ��ʵ����ʾ��ͼ�����߱�ʾ���ı�ijһ��Ӧ����ʱ��H2�����ʵ�����ʱ��ı仯������˵����ȷ���ǣ��������鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| 1 | 2 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
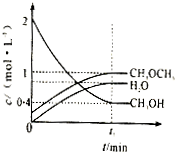 ��1������ˮú���ϳɶ����ѣ�CH3OCH3�����Ȼ�ѧ����ʽΪ��3H2��g��+3CO��g��?CH3OCH3��g��+CO2��g������H=-274KJ/mol���÷�Ӧ��һ�������µ��ܱ������дﵽƽ���Ϊͬʱ��߷�Ӧ���ʺͶ����ѵIJ��ʣ����Բ�ȡ�Ĵ�ʩ��
��1������ˮú���ϳɶ����ѣ�CH3OCH3�����Ȼ�ѧ����ʽΪ��3H2��g��+3CO��g��?CH3OCH3��g��+CO2��g������H=-274KJ/mol���÷�Ӧ��һ�������µ��ܱ������дﵽƽ���Ϊͬʱ��߷�Ӧ���ʺͶ����ѵIJ��ʣ����Բ�ȡ�Ĵ�ʩ���鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com