��2013?���ݶ�ģ����ҵ��������Ĺ������£�����ˮ
��Һ
����
����
���������գ�
��1������ˮ������������Mg
2+��Ca
2+�������������A��B�����ʣ�A��Դ��ʯ��Ҥ�������������B�Ļ�ѧʽΪ
Na2CO3
Na2CO3
��
��2��ʵ����ģ������Һ�Ʊ�������װ�����£�

��ͼ1��װ�ú�ͼ2��װ�õ����ӷ���Ϊa��
d
d
��b��
e
e
��f��c��
��ͼ2���Լ�ƿ�ڷ����Ļ�ѧ��Ӧ����ʽΪ
NaCl+NH3+CO2+H2O=NaHCO3��+NH4Cl
NaCl+NH3+CO2+H2O=NaHCO3��+NH4Cl
��
��ʵ����Ҫ��ͨ���NH
3����֮����ͨ��CO
2���壬����ͨ���NH
3�ѹ�����ʵ�������
��պ��Ũ����IJ����������ܿ�f�����а������ɣ�˵����������������ʪ��ĺ�ɫʯ����ֽ�����ܿ�f������ֽ������˵����������
��պ��Ũ����IJ����������ܿ�f�����а������ɣ�˵����������������ʪ��ĺ�ɫʯ����ֽ�����ܿ�f������ֽ������˵����������
��
��3������=5\*MERGEFORMAT �����պ�Ĵ����к���δ�ֽ��̼�����ƣ�ijͬѧ��ȡ�ô�����Ʒm g���ٳ�ּ������������ٱ仯ʱ�Ƶ�ʣ����������Ϊn g������Ʒ��̼���Ƶ���������Ϊ
��
��4������25���£�0.1mol/LNH
3?H
2O��Һ��0.1mol/LNH
4Cl��Һ����������Һ�������ϲ����Һ��pH=9������˵����ȷ����
cd
cd
������ţ���
A��0.1mol/L NH
4Cl��Һ���Ϻ���Һ�е������ӵ��������Ŀ����ͬ
b����Ϻ����Һ�У�c��NH
3?H
2O����c��Cl
-����c��NH
4+����c��OH
-����c��H
+��
c���������֪��NH
3?H
2O�ĵ���̶ȴ���ͬŨ�ȵ�NH
4Cl��ˮ��̶�
d�����ǰ������Һ��pH֮�ʹ���14��




 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�

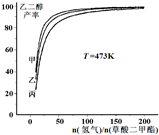 ��������/n�������������]��ѹǿ�ı仯��ϵ�������������߷ֱ��ʾ��ϵѹǿΪ1.5MPa��2.5MPa��3.5MPa�������������Ӧ��ѹǿ��P���ף�=
��������/n�������������]��ѹǿ�ı仯��ϵ�������������߷ֱ��ʾ��ϵѹǿΪ1.5MPa��2.5MPa��3.5MPa�������������Ӧ��ѹǿ��P���ף�=
