
ijѧ����0.1000 mol/L��KOH��Һ�ζ�δ֪Ũ�ȵ�������Һ��������ɷֽ�Ϊ���¼�����
(A)��ȡ20.00 mL�����������Һע��ྻ����ƿ��������2��3�η�̪
(B)�ñ���Һ��ϴ�ζ���2��3��
(C)��ʢ�б���Һ�ļ�ʽ�ζ��̶ܹ��ã�����Һ��ʹ�ζ��ܼ��������Һ
(D)ȡ��KOH��Һע���ʽ�ζ�����0�̶�����2��3 cm
(E)����Һ����0��0�̶����£����¶���
(F)����ƿ���ڵζ��ܵ����棬�ñ�KOH��Һ�ζ����յ㣬���µζ���Һ��Ŀ̶�
���������գ�
(1)��ȷ������˳����(�������ĸ��д)________.
(2)����(B)������Ŀ����________��
(3)����(A)����֮ǰ�������ô���Һ��ϴ��ƿ����Բⶨ�����Ӱ����(��ƫ��ƫС�����䣬��ͬ)________��
(4)ʵ���������ֿ���________(����������λ)���۾�ע��________��ֱ���ζ��յ㣮�жϵ����յ��������________��
(5)����ȡһ������KOH����(������NaOH)���Ʊ���Һ�������ζ��������ᣬ��Բⶨ�����Ӱ����________��
(6)�ζ������������ӹ۲�ζ�����Һ��̶ȣ���Եζ������Ӱ����________��

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��12�֣�ijѧ����0.1000 mol��L��1������������Һ�ζ�δ֪Ũ�ȵ�������Һ��������ɷֽ�Ϊ���¼�����
A��ȡ20.00 mL����������Һע��ྻ����ƿ�У�������2��3�η�̪��Һ
B���ñ�����������Һ��ϴ�ζ���2��3��
C����ʢ�б�����������Һ�ļ�ʽ�ζ��̶ܹ��ã����ڵζ��ܼ���ʹ֮������Һ
D��ȡ������������Һע���ʽ�ζ�������0���̶�����2��3mL��
E������Һ������0����0���̶����£����¶�����Ϊ3.10mL
F������ƿ���ڵζ������棬�ñ�NaOH��Һ�ζ����յ㲢���µζ��ܵĶ�����
�ش��������⣺
��1����ȷ�IJ���˳���ǣ�B�� ��F������ţ���
��2������B���������Ŀ���� ��
��3������A�������֮ǰ�������ô�����Һ��ϴ��ƿ����Եζ������Ӱ��
�� ���ƫ��ƫС������Ӱ�족����
��4���жϵ���ζ��յ��ʵ�������� ��
��5�����ζ�����ʱ���ζ���Һ����ͼ��ʾ�����յ����Ϊ mL��
��6�����ݶ���������ô���Һ��Ũ��Ϊ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��12�֣�ijѧ����0.1000 mol��L��1������������Һ�ζ�δ֪Ũ�ȵ�������Һ��������ɷֽ�Ϊ���¼�����
A��ȡ20.00 mL����������Һע��ྻ����ƿ�У�������2��3�η�̪��Һ
B���ñ�����������Һ��ϴ�ζ���2��3��
C����ʢ�б�����������Һ�ļ�ʽ�ζ��̶ܹ��ã����ڵζ��ܼ���ʹ֮������Һ
D��ȡ������������Һע���ʽ�ζ�������0���̶�����2��3mL��
E������Һ������0����0���̶����£����¶�����Ϊ3.10mL
F������ƿ���ڵζ������棬�ñ�NaOH��Һ�ζ����յ㲢���µζ��ܵĶ�����
�ش��������⣺
��1����ȷ�IJ���˳���ǣ�B�� ��F������ţ���
��2������B���������Ŀ���� ��
��3������A�������֮ǰ�������ô�����Һ��ϴ��ƿ����Եζ������Ӱ��
�� ���ƫ��ƫС������Ӱ�족����
��4���жϵ���ζ��յ��ʵ�������� ��
��5�����ζ�����ʱ���ζ���Һ����ͼ��ʾ�����յ����Ϊ mL��
��6�����ݶ���������ô���Һ��Ũ��Ϊ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012-2013ѧ�꽭��ʡ��Ǩ�������غ�����ѧ�߶���ѧ�����п���ѧ������������ ���ͣ�ʵ����
ijѧ����0.1000 mol��L��1������������Һ�ζ�δ֪Ũ�ȵ�������Һ��������ɷֽ�Ϊ���¼�����
A��ȡ20.00 mL����������Һע��ྻ����ƿ�У�������2��3�η�̪��Һ
B���ñ�����������Һ��ϴ�ζ���2��3��
C����ʢ�б�����������Һ�ļ�ʽ�ζ��̶ܹ��ã����ڵζ��ܼ���ʹ֮������Һ
D��ȡ������������Һע���ʽ�ζ�������0���̶�����2��3mL��
E������Һ������0����0���̶����£����¶�����Ϊ3.10mL
F������ƿ���ڵζ��ܵ����棬�ñ�NaOH��Һ�ζ����յ㲢���µζ��ܵĶ������ش��������⣺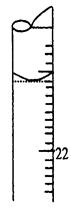
��1����ȷ�IJ���˳���ǣ�B�� ��F������ţ���
��2������B���������Ŀ���� ��
��3������A�������֮ǰ�������ô�����Һ��ϴ��ƿ����Եζ������Ӱ��
�� ���ƫ��ƫС������Ӱ�족����
��4���жϵ���ζ��յ��ʵ�������� ��
��5�����ζ�����ʱ���ζ���Һ����ͼ��ʾ�����յ����Ϊ mL��
��6�����ݶ���������ô���Һ��Ũ��Ϊ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2014�콭��ʡ��Ǩ�и߶���ѧ�����п���ѧ���������棩 ���ͣ�ʵ����
ijѧ����0.1000 mol��L��1������������Һ�ζ�δ֪Ũ�ȵ�������Һ��������ɷֽ�Ϊ���¼�����
A��ȡ20.00 mL����������Һע��ྻ����ƿ�У�������2��3�η�̪��Һ
B���ñ�����������Һ��ϴ�ζ���2��3��
C����ʢ�б�����������Һ�ļ�ʽ�ζ��̶ܹ��ã����ڵζ��ܼ���ʹ֮������Һ
D��ȡ������������Һע���ʽ�ζ�������0���̶�����2��3mL��
E������Һ������0����0���̶����£����¶�����Ϊ3.10mL
F������ƿ���ڵζ��ܵ����棬�ñ�NaOH��Һ�ζ����յ㲢���µζ��ܵĶ������ش��������⣺
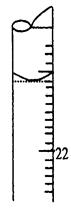
��1����ȷ�IJ���˳���ǣ�B�� ��F������ţ���
��2������B���������Ŀ���� ��
��3������A�������֮ǰ�������ô�����Һ��ϴ��ƿ����Եζ������Ӱ��
�� ���ƫ��ƫС������Ӱ�족����
��4���жϵ���ζ��յ��ʵ�������� ��
��5�����ζ�����ʱ���ζ���Һ����ͼ��ʾ�����յ����Ϊ mL��
��6�����ݶ���������ô���Һ��Ũ��Ϊ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2013���㽭ʡ�����и߶����п��Ի�ѧ�Ծ��������棩 ���ͣ������
��12�֣�ijѧ����0.1000 mol��L��1������������Һ�ζ�δ֪Ũ�ȵ�������Һ��������ɷֽ�Ϊ���¼�����
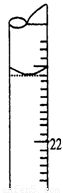
A��ȡ20.00 mL����������Һע��ྻ����ƿ�У�������2��3�η�̪��Һ
B���ñ�����������Һ��ϴ�ζ���2��3��
C����ʢ�б�����������Һ�ļ�ʽ�ζ��̶ܹ��ã����ڵζ��ܼ���ʹ֮������Һ
D��ȡ������������Һע���ʽ�ζ�������0���̶�����2��3mL��
E������Һ������0����0���̶����£����¶�����Ϊ3.10mL
F������ƿ���ڵζ������棬�ñ�NaOH��Һ�ζ����յ㲢���µζ��ܵĶ�����
�ش��������⣺
��1����ȷ�IJ���˳���ǣ�B�� ��F������ţ���
��2������B���������Ŀ���� ��
��3������A�������֮ǰ�������ô�����Һ��ϴ��ƿ����Եζ������Ӱ��
�� ���ƫ��ƫС������Ӱ�족����
��4���жϵ���ζ��յ��ʵ�������� ��
��5�����ζ�����ʱ���ζ���Һ����ͼ��ʾ�����յ����Ϊ mL��
��6�����ݶ���������ô���Һ��Ũ��Ϊ ��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com