
SO2�Ĵ������ǹ�ҵ��ȡ����Ĺؼ�����֮һ���÷�Ӧ�Ļ�ѧ����ʽΪ��
2SO2��O2 2SO3
2SO3  ��H<0��
��H<0��
��ش��������⣺
��1���жϸ÷�Ӧ�ﵽƽ��״̬�ı�־��_____________������ĸ����
a��SO2��SO3Ũ�����
b��SO2�ٷֺ������ֲ���
c�������������ѹǿ����
d��SO3������������SO2�������������
��2������ ��Ӧ����ƽ��״̬ʱ�����������������£����д�ʩ�����������SO2ƽ��ת���ʵ���_____________������ĸ����
��Ӧ����ƽ��״̬ʱ�����������������£����д�ʩ�����������SO2ƽ��ת���ʵ���_____________������ĸ����
a����װ�����ٳ���N2 b����װ�����ٳ���O2
c���ı䷴Ӧ�Ĵ��� d�������¶�
��3����0.050 mol SO2��g����0.030 mol O2��g�������ݻ�Ϊ1 L���ܱ������У���һ�������´ﵽƽ�⣬���c��SO3����0.040 mol/L������������·�Ӧ��ƽ�ⳣ��K��SO2��ת���ʣ�����д��������̣���
��ƽ�ⳣ��K��____________________��
��ƽ��ʱ��SO2��ת���ʦ���SO2����____________________��
��4��SO2β�����ñ���Na2SO3��Һ���գ�����SO2������Ⱦ���ɵõ���Ҫ�Ļ���ԭ��NaHSO3����֪NaHSO3��Һͬʱ������������ƽ�⣺��HSO
 SO
SO ��H������HSO
��H������HSO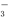 ��H2O
��H2O H2SO3��OH���������£�0.1 mol/L NaH
H2SO3��OH���������£�0.1 mol/L NaH SO3��Һ��pH<7�������Һ��c��H2SO3��______________c��SO
SO3��Һ��pH<7�������Һ��c��H2SO3��______________c��SO �����>������������<������
�����>������������<������

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ��2016-2017ѧ��㶫ʡ��ͷ�и߶���ѧҵˮƽ���Ի�ѧ���������棩 ���ͣ�ѡ����
����ˮ���������ж��֣�����ɱ������ǿ���ֲ�Ӱ��ˮ�ʵ������������ǣ� ��
A������(03) B��Ư�� C���� D������ �� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2016-2017ѧ�긣��ʡ������У��һ��ѧ������������ѧ�Ծ��������棩 ���ͣ�ѡ����
�����������ͬ���ܱ������зֱ����Ne��H2��O2�������壬�����ǵ��¶Ⱥ��ܶȶ���
ͬʱ�������������ѹǿ��p���ɴ�С��˳���ǣ� ��
A��p��Ne��>p��H2��>p��02�� B��p��O2��>p��Ne��>p��H2��
C�� p��H2��>p��O2��>p��Ne�� D��p��H2��>p��Ne��>p��O2��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2016-2017ѧ�����ʦ���и߶�����������ѧ�Ծ��������棩 ���ͣ������
������ͼ�У�PΪһ�����ɻ����Ļ��� ���ر�K���ֱ�������A��B�и�����1 mol X��1 mol Y����ʼʱ��VA��a L��VB��0.8a L����ͨ�ܵ�������Բ��ƣ�������ͬ�¶Ⱥ��д������ڵ������£��������и��Է���
���ر�K���ֱ�������A��B�и�����1 mol X��1 mol Y����ʼʱ��VA��a L��VB��0.8a L����ͨ�ܵ�������Բ��ƣ�������ͬ�¶Ⱥ��д������ڵ������£��������и��Է��� ������Ӧ��3X��g����3Y��g��
������Ӧ��3X��g����3Y��g�� 2Z��g����2W��g������ƽ��ʱ��VB��0.6a L���ش��������⣺
2Z��g����2W��g������ƽ��ʱ��VB��0.6a L���ش��������⣺

��1���ﵽƽ��ʱB��X��ת����Ϊ________________��
��2��ƽ��ʱA��B�л�������ƽ����Է��������Ĺ�ϵ�ǣ�MA____________MB���>������������<������
��3����K��һ��ʱ���Ӧ�ٴδﵽƽ�⣬��B�����Ϊ____________L��
����ҵ�в����ʵ��Ĵ������÷�ӦCO��g����2H2��g�� CH3OH��g������ȡ�״���
CH3OH��g������ȡ�״���
��4��ά����ϵ��ѹǿһ�����¶�ΪTʱ�������Ϊ2.0 L�ķ�������ڳ���0.6 mol CO��0.4 mol H2���ﵽƽ��ʱ����0.15 mol CH3OH��g������÷�Ӧƽ�ⳣ��K��________��������λС��������ʱ����������ͨ��0.7 mol CO���壬���ƽ�⽫____________��������������������ƶ���
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2016-2017ѧ�����ʦ���и߶�����������ѧ�Ծ��������棩 ���ͣ�ѡ����
��֪3.6 g̼��6.4 g��������ȼ�գ�����Ӧ��ľ������ų�X kJ��������֪����̼��ȼ����Ϊ Y kJ/mol����1 mol C��O2��Ӧ����CO�ķ�Ӧ�Ȧ�HΪ____________
Y kJ/mol����1 mol C��O2��Ӧ����CO�ķ�Ӧ�Ȧ�HΪ____________
A����Y kJ/mol B������10X��Y�� kJ/mol
C������5X��0.5Y�� kJ/mol D������10X��Y�� kJ/mol
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2016-2017ѧ�����ʦ���и߶�����������ѧ�Ծ��������棩 ���ͣ�ѡ����
������O2���ͳ�����O3������Ԫ�ص�����ͬ�������壬��֪�Ȼ�ѧ����ʽ��
4Al��s����3O2��g��=2Al2O3��s�� ��H1��
4Al��s����2O3��g��=2Al2O3��s�� ��H2��
3O2��g��=2O3��g�� ��H3����
A����H1����H2����H3 B����H1����H2����H3
C����H2����H1����H3 D����H2����H1����H3��0
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2016-2017ѧ�����ʦ���и߶�����������ѧ�Ծ��������棩 ���ͣ�ѡ����
�Դ���ĵ���ƽ�ⲻ����Ӱ�������
A. B��HBr C.
B��HBr C. D��CH3COO��
D��CH3COO��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2016-2017ѧ�����ʡ��һ�����л�ѧ�Ծ��������棩 ���ͣ�ѡ����
��֪��2Fe + 3Br2��2FeBr3��Fe2+�Ļ�ԭ�Դ���Br��������16.8 g����0.3 mol Br2��Ӧ�����ˮ�õ�������Һ��ͨ�� a mol Cl2��������������ȷ����
a mol Cl2��������������ȷ����
A����a=0.1ʱ�������ķ�ӦΪ2Fe2++Cl2��2Fe3++2Cl��
B����a=0.45ʱ�������ķ�ӦΪ2Fe2++4Br��+3Cl 2��2Fe3++2Br2+6Cl��
2��2Fe3++2Br2+6Cl��
C������Һ��Br����һ�뱻����ʱ��c��Fe3+��:c��Br����:c��Cl������1:1:3
D����0��a��0.15ʱ����Һ��ʼ������2c��Fe2+��+3c��Fe3+��+c��H+����c��C l����+c��Br����+c��OH����
l����+c��Br����+c��OH����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2016-2017ѧ�����ʡۣ��һ�ߡ�Ҷ�ض��ߵ���У�߶������л�ѧ���������棩 ���ͣ�ѡ����
�������ӷ���ʽ��д��ȷ���ǣ� ��
A.NaHSˮ�ⷴӦ��HS��+H2O H3O++S2��
H3O++S2��
B������������ˮ�������õ�ԭ��Al3++3H2O Al(OH)3(����)+3H+
Al(OH)3(����)+3H+
C������������Һ��ϡ���ᷴӦ��
D��̼��������Һ�м������Ba(OH)2��Һ��2HCO3��+Ba2++2OH��=BaCO3��+CO32��+2H2O
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com