
ЁОЬтФПЁПЛЏКЯЮяGЪЧКЯГЩПЙаФТЩЪЇГЃвЉЮяОіФЮДяТЁЕФвЛжжжаМфЬхЃЌПЩЭЈЙ§вдЯТЗНЗЈКЯГЩЃК
ЧыЛиД№ЯТСаЮЪЬтЃК
ЃЈ1ЃЉRЕФУћГЦЪЧ_______________ЃЛNжаКЌгаЕФЙйФмЭХЪ§ФПЪЧ______ЁЃ
ЃЈ2ЃЉMЁњNЗДгІЙ§ГЬжаK2CO3ЕФзїгУЪЧ____________________________________ЁЃ
ЃЈ3ЃЉHЁњGЕФЗДгІРраЭЪЧ______________ЁЃ
ЃЈ4ЃЉHЕФЗжзгЪН________________ЁЃ
ЃЈ5ЃЉаДГіQЁњHЕФЛЏбЇЗНГЬЪНЃК___________________________________ЁЃ
ЃЈ6ЃЉTгыRзщГЩдЊЫижжРрЯрЭЌЃЌЗћКЯЯТСаЬѕМўTЕФЭЌЗжвьЙЙЬхга_____жжЁЃ
ЂйгыRОпгаЯрЭЌЙйФмЭХЃЛЂкЗжзгжаКЌгаБНЛЗЃЛЂлTЕФЯрЖдЗжзгжЪСПБШRЖр14
ЦфжадкКЫДХЙВеёЧтЦзЩЯга5зщЗхЧвЗхЕФУцЛ§БШЮЊ1:1:2:2:2ЕФНсЙЙМђЪНга___________ЁЃ
ЃЈ7ЃЉвд1ЃЌ5-ЮьЖўДМ(![]() ) КЭБНЮЊдСЯ(ЦфЫћЮоЛњЪдМСздбЁ)КЯГЩ
) КЭБНЮЊдСЯ(ЦфЫћЮоЛњЪдМСздбЁ)КЯГЩ![]() ЃЌЩшМЦКЯГЩТЗЯпЃК_________________________________________ЁЃ
ЃЌЩшМЦКЯГЩТЗЯпЃК_________________________________________ЁЃ
ЁОД№АИЁПСкєЧЛљБНМзШЉ(Лђ2-єЧЛљБНМзШЉ) 4 ЯћКФВњЩњЕФHClЃЌЬсИпгаЛњЮяNЕФВњТЪ ШЁДњЗДгІ C15H18N2O3 ![]() +
+![]() Ёњ2HCl+
Ёњ2HCl+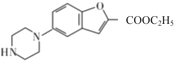 17
17  ЁЂ
ЁЂ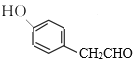
ЁОНтЮіЁП
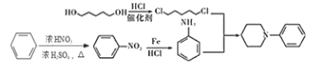
RдквЛЖЈЬѕМўЯТЗДгІЩњГЩMЃЌMдкЬМЫсМизїгУЯТЃЌгыClCH2COOC2H5ЗДгІЩњГЩNЃЌИљОнNЕФНсЙЙМђЪНЃЌMЕФНсЙЙМђЪНЮЊ ЃЌИљОнRКЭMЕФЗжзгЪНЃЌRдкХЈСђЫсЁЂХЈЯѕЫсзїгУЯТЗЂЩњШЁДњЗДгІЩњГЩMЃЌдђRЕФНсЙЙМђЪНЮЊ
ЃЌИљОнRКЭMЕФЗжзгЪНЃЌRдкХЈСђЫсЁЂХЈЯѕЫсзїгУЯТЗЂЩњШЁДњЗДгІЩњГЩMЃЌдђRЕФНсЙЙМђЪНЮЊ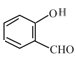 ЃЌNдквЛЖЈЬѕМўЯТЗДгІЩњГЩPЃЌPдкFe/HClзїгУЯТЩњГЩQЃЌQгы
ЃЌNдквЛЖЈЬѕМўЯТЗДгІЩњГЩPЃЌPдкFe/HClзїгУЯТЩњГЩQЃЌQгы![]() ЗДгІЩњГЩHЃЌИљОнHЕФНсЙЙМђЪНКЭвбжЊаХЯЂЃЌдђQЕФНсЙЙМђЪНЮЊ
ЗДгІЩњГЩHЃЌИљОнHЕФНсЙЙМђЪНКЭвбжЊаХЯЂЃЌдђQЕФНсЙЙМђЪНЮЊ![]() ЃЌPЕФНсЙЙМђЪНЮЊЃК
ЃЌPЕФНсЙЙМђЪНЮЊЃК ЃЛHдк(Boc)2OзїгУЯТЗДгІЩњГЩGЃЌОнДЫЗжЮіНтД№ЁЃ
ЃЛHдк(Boc)2OзїгУЯТЗДгІЩњГЩGЃЌОнДЫЗжЮіНтД№ЁЃ
(1)ИљОнЗжЮіЃЌ RЕФНсЙЙМђЪНЮЊ ЃЌУћГЦЪЧСкєЧЛљБНМзШЉ(Лђ2-єЧЛљБНМзШЉ)ЃЛИљОнNЕФНсЙЙМђЪНЃЌЦфжаКЌгаЕФЙйФмЭХгаУбЛљЁЂѕЅЛљЁЂЯѕЛљЁЂШЉЛљЃЌЙВ4жжЙйФмЭХЃЛ
ЃЌУћГЦЪЧСкєЧЛљБНМзШЉ(Лђ2-єЧЛљБНМзШЉ)ЃЛИљОнNЕФНсЙЙМђЪНЃЌЦфжаКЌгаЕФЙйФмЭХгаУбЛљЁЂѕЅЛљЁЂЯѕЛљЁЂШЉЛљЃЌЙВ4жжЙйФмЭХЃЛ
(2)MдкЬМЫсМизїгУЯТЃЌгыClCH2COOC2H5ЗДгІЩњГЩNЃЌИљОнNЕФНсЙЙМђЪНЃЌMЕФНсЙЙМђЪНЮЊ ЃЌИљОнНсЙЙБфЛЏЃЌMЕНNЗЂЩњШЁДњЗДгІЩњГЩNЕФЭЌЪБЛЙЩњГЩHClЃЌМгШыK2CO3ПЩЯћКФВњЩњЕФHClЃЌЬсИпгаЛњЮяNЕФВњТЪЃЛ
ЃЌИљОнНсЙЙБфЛЏЃЌMЕНNЗЂЩњШЁДњЗДгІЩњГЩNЕФЭЌЪБЛЙЩњГЩHClЃЌМгШыK2CO3ПЩЯћКФВњЩњЕФHClЃЌЬсИпгаЛњЮяNЕФВњТЪЃЛ
(3)Qгы![]() ЗДгІЩњГЩHЃЌQЕФНсЙЙМђЪНЮЊ
ЗДгІЩњГЩHЃЌQЕФНсЙЙМђЪНЮЊ![]() ЃЌQжаАБЛљЩЯЕФЧтдзгБЛШЁДњЃЌHЁњGЕФЗДгІРраЭЪЧШЁДњЗДгІЃЛ
ЃЌQжаАБЛљЩЯЕФЧтдзгБЛШЁДњЃЌHЁњGЕФЗДгІРраЭЪЧШЁДњЗДгІЃЛ
(4)НсЙЙЪНжаЃЌЮДБъзЂдЊЫиЗћКХЕФУПИіНкЕуЮЊЬМдзгЃЌУПИіЬМдзгСЌНг4ИіМќЃЌВЛзуМќгЩЧтдзгВЙЦыЃЌHЕФЗжзгЪНC15H18N2O3ЃЛ
(5)Qгы![]() ЗДгІЩњГЩHЃЌQЕФНсЙЙМђЪНЮЊ
ЗДгІЩњГЩHЃЌQЕФНсЙЙМђЪНЮЊ![]() ЃЌQЁњHЕФЛЏбЇЗНГЬЪНЃК
ЃЌQЁњHЕФЛЏбЇЗНГЬЪНЃК![]() +
+![]() Ёњ2HCl+
Ёњ2HCl+ ЃЛ
ЃЛ
(6) RЕФНсЙЙМђЪНЮЊ ЃЌTгыRзщГЩдЊЫижжРрЯрЭЌЃЌЂйгыRОпгаЯрЭЌЙйФмЭХЃЌЫЕУїTЕФНсЙЙжаКЌгаєЧЛљКЭШЉЛљЃЛЂкЗжзгжаКЌгаБНЛЗЃЛЂлTЕФЯрЖдЗжзгжЪСПБШRЖр14ЃЌМДTБШRжаЖрвЛИі-CH2-ЃЌШєTЪЧгЩ
ЃЌTгыRзщГЩдЊЫижжРрЯрЭЌЃЌЂйгыRОпгаЯрЭЌЙйФмЭХЃЌЫЕУїTЕФНсЙЙжаКЌгаєЧЛљКЭШЉЛљЃЛЂкЗжзгжаКЌгаБНЛЗЃЛЂлTЕФЯрЖдЗжзгжЪСПБШRЖр14ЃЌМДTБШRжаЖрвЛИі-CH2-ЃЌШєTЪЧгЩ КЭ-CH3ЙЙГЩЃЌдђСЌНгЗНЪНЮЊ
КЭ-CH3ЙЙГЩЃЌдђСЌНгЗНЪНЮЊ ЃЛШєTЪЧгЩ
ЃЛШєTЪЧгЩ КЭ-CH3ЙЙГЩЃЌдђСЌНгЗНЪНЮЊ
КЭ-CH3ЙЙГЩЃЌдђСЌНгЗНЪНЮЊ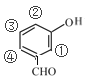 ЃЛШєTЪЧгЩ
ЃЛШєTЪЧгЩ КЭ-CH3ЙЙГЩЃЌдђСЌНгЗНЪНЮЊ
КЭ-CH3ЙЙГЩЃЌдђСЌНгЗНЪНЮЊ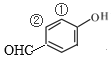 ЃЛШєTЪЧгЩ
ЃЛШєTЪЧгЩ КЭ-CHOЙЙГЩЃЌдђСЌНгЗНЪНЮЊ
КЭ-CHOЙЙГЩЃЌдђСЌНгЗНЪНЮЊ ЃЛШєTЪЧгЩ
ЃЛШєTЪЧгЩ КЭ-OHЙЙГЩЃЌдђСЌНгЗНЪНЮЊ
КЭ-OHЙЙГЩЃЌдђСЌНгЗНЪНЮЊ ЃЛШєTЪЧгЩ
ЃЛШєTЪЧгЩ![]() КЭ-CH(OH)CHOЙЙГЩЃЌдђСЌНгЗНЪНЮЊ
КЭ-CH(OH)CHOЙЙГЩЃЌдђСЌНгЗНЪНЮЊ![]() ЃЌЗћКЯЯТСаЬѕМўTЕФЭЌЗжвьЙЙЬхга17жжЃЛЦфжадкКЫДХЙВеёЧтЦзЩЯга5зщЗхЧвЗхЕФУцЛ§БШЮЊ1:1:2:2:2ЃЌЫЕУїЗжзгжаКЌга5жжВЛЭЌЛЗОГЕФЧтдзгЃЌНсЙЙМђЪНга
ЃЌЗћКЯЯТСаЬѕМўTЕФЭЌЗжвьЙЙЬхга17жжЃЛЦфжадкКЫДХЙВеёЧтЦзЩЯга5зщЗхЧвЗхЕФУцЛ§БШЮЊ1:1:2:2:2ЃЌЫЕУїЗжзгжаКЌга5жжВЛЭЌЛЗОГЕФЧтдзгЃЌНсЙЙМђЪНга ЁЂ
ЁЂ ЃЛ
ЃЛ
(7)вд1ЃЌ5-ЮьЖўДМ(![]() ) КЭБНЮЊдСЯЃЌвРОнСїГЬЭМжаQЁњFЕФаХЯЂЃЌашНЋвд1ЃЌ5-ЮьЖўДМзЊЛЏЮЊ1ЃЌ5-ЖўТШЮьЭщЃЌНЋБОзЊЛЏЮЊЯѕЛљБНЃЌдйНЋЯѕЛљБНзЊЛЏЮЊБНАЗЃЌвдБугкКЯГЩФПБъгаЛњЮя
) КЭБНЮЊдСЯЃЌвРОнСїГЬЭМжаQЁњFЕФаХЯЂЃЌашНЋвд1ЃЌ5-ЮьЖўДМзЊЛЏЮЊ1ЃЌ5-ЖўТШЮьЭщЃЌНЋБОзЊЛЏЮЊЯѕЛљБНЃЌдйНЋЯѕЛљБНзЊЛЏЮЊБНАЗЃЌвдБугкКЯГЩФПБъгаЛњЮя![]() ЃЌЩшМЦКЯГЩТЗЯпЃК
ЃЌЩшМЦКЯГЩТЗЯпЃК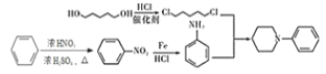 ЁЃ
ЁЃ



| ФъМЖ | ИпжаПЮГЬ | ФъМЖ | ГѕжаПЮГЬ |
| ИпвЛ | ИпвЛУтЗбПЮГЬЭЦМіЃЁ | ГѕвЛ | ГѕвЛУтЗбПЮГЬЭЦМіЃЁ |
| ИпЖў | ИпЖўУтЗбПЮГЬЭЦМіЃЁ | ГѕЖў | ГѕЖўУтЗбПЮГЬЭЦМіЃЁ |
| ИпШ§ | ИпШ§УтЗбПЮГЬЭЦМіЃЁ | ГѕШ§ | ГѕШ§УтЗбПЮГЬЭЦМіЃЁ |
ПЦФПЃКИпжаЛЏбЇ РДдДЃК ЬтаЭЃК
ЁОЬтФПЁПЦћГЕЮВЦјжаNOВњЩњЕФЗДгІЮЊЃКN2(g)+O2(g)![]() 2NO(g)ЃЌвЛЖЈЬѕМўЯТЃЌЕШЮяжЪЕФСПЕФN2(g)КЭO2(g)дкКуШнУмБеШнЦїжаЗДгІЃЌЯТЭМЧњЯпaБэЪОИУЗДгІдкЮТЖШTЯТN2ЕФХЈЖШЫцЪБМфЕФБфЛЏЃЌЧњЯпbБэЪОИУЗДгІдкФГвЛЦ№ЪМЗДгІЬѕМўИФБфЪБN2ЕФХЈЖШЫцЪБМфЕФБфЛЏЁЃЯТСаа№Ъіе§ШЗЕФЪЧЃК
2NO(g)ЃЌвЛЖЈЬѕМўЯТЃЌЕШЮяжЪЕФСПЕФN2(g)КЭO2(g)дкКуШнУмБеШнЦїжаЗДгІЃЌЯТЭМЧњЯпaБэЪОИУЗДгІдкЮТЖШTЯТN2ЕФХЈЖШЫцЪБМфЕФБфЛЏЃЌЧњЯпbБэЪОИУЗДгІдкФГвЛЦ№ЪМЗДгІЬѕМўИФБфЪБN2ЕФХЈЖШЫцЪБМфЕФБфЛЏЁЃЯТСаа№Ъіе§ШЗЕФЪЧЃК
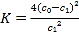
A. ЮТЖШTЯТЃЌИУЗДгІЕФЦНКтГЃЪ§K=![]()
B. ЮТЖШTЯТЃЌЫцзХЗДгІЕФНјааЃЌЛьКЯЦјЬхЕФУмЖШМѕаЁ
C. ЧњЯпbЖдгІЕФЬѕМўИФБфПЩФмЪЧМгШыСЫДпЛЏМС
D. ШєЧњЯпbЖдгІЕФЬѕМўИФБфЪЧЮТЖШЃЌПЩХаЖЯИУЗДгІЕФЁїHЃМ0
ВщПДД№АИКЭНтЮі>>
ПЦФПЃКИпжаЛЏбЇ РДдДЃК ЬтаЭЃК
ЁОЬтФПЁПСДзДгаЛњЮяAЪЧвЛжжЪГгУаЭЯуОЋЃЌдквЛЖЈЬѕМўЯТгаШчЯТБфЛЏЃК
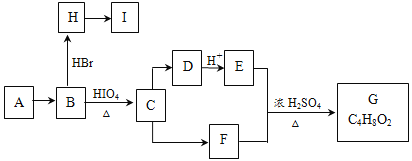
вбжЊЃКЃЈ1ЃЉ
ЃЈ2ЃЉAКЭGЛЅЮЊЭЌЗжвьЙЙЬхЃЌAВЛФмЪЙBr2ЕФCCl4ШмвКЭЪЩЋЁЃBКЭFжаЫљКЌЙйФмЭХЕФРраЭЯрЭЌЁЃ
ЭъГЩЯТСаЬюПеЃК
ЃЈ1ЃЉFЕФЗжзгЪНЮЊ________________ЃЛCЁњDЕФЗДгІРраЭЪЧ__________________________ЁЃ
ЃЈ2ЃЉAЕФНсЙЙМђЪНЮЊ________________________________ЁЃ
ЃЈ3ЃЉШєCжаЛьгаЩйСПЕФEЃЌЧыаДГіЯргІЕФГ§дгЪдМСКЭЗжРыЗНЗЈЃК________________ЁЃ
ЃЈ4ЃЉIжаЫљгаЬМдзгОљдквЛЬѕжБЯпЩЯЃЌHзЊЛЏЮЊIЕФЛЏбЇЗНГЬЪНЮЊ:______________________ЁЃ
ЃЈ5ЃЉXЪЧAЕФвЛжжЭЌЗжвьЙЙЬхЃЌ1mol XдкHIO4МгШШЬѕМўЯТЭъШЋЗДгІЃЌПЩвдЩњГЩ1molЮожЇСДгаЛњЮяЃЌдђXЕФНсЙЙМђЪНЮЊ_________________ЁЃ
ЃЈ6ЃЉЩшМЦга1-ЖЁЯЉЮЊдСЯЃЌКЯГЩHЕФКЯГЩТЗЯпЁЃЃЈКЯГЩТЗЯпГЃгУЕФБэЪОЗНЪНЮЊЃКМз![]() ввЁЁ
ввЁЁ![]() ФПБъВњЮяЃЉ______________________________________
ФПБъВњЮяЃЉ______________________________________
ВщПДД№АИКЭНтЮі>>
ПЦФПЃКИпжаЛЏбЇ РДдДЃК ЬтаЭЃК
ЁОЬтФПЁПFeCl3ЪЧжабЇЪЕбщЪвГЃгУЕФЪдМСЁЃ
ЃЈ1ЃЉаДГіТШЛЏЬњдкЫЎжаЕФЕчРыЗНГЬЪНЃК_________ЁЃ
ЃЈ2ЃЉРћгУТШЛЏЬњШмвКжЦБИЧтбѕЛЏЬњНКЬхЁЃ
ЂйЯТСажЦБИЧтбѕЛЏЬњНКЬхЕФВйзїЗНЗЈе§ШЗЕФЪЧ_______ЃЈЬюзжФИЃЉЁЃ
A.ЯђБЅКЭТШЛЏЬњШмвКжаЕЮМгЪЪСПЕФЧтбѕЛЏФЦЯЁШмвК
B.МгШШжѓЗаТШЛЏЬњБЅКЭШмвК
C.дкАБЫЎжаЕЮМгТШЛЏЬњХЈШмвК
D.дкЗаЫЎжаЕЮМгБЅКЭТШЛЏЬњШмвКЃЌжѓЗажСГіЯжКьКжЩЋвКЬх
ЂкЧјБ№ТШЛЏЬњШмвККЭЧтбѕЛЏЬњНКЬхЕФЗНЗЈЪЧ___________ЁЃ
ЃЈ3ЃЉЮЊСЫЬНОПРызгЗДгІЕФБОжЪЃЌЩшМЦШчЯТЪЕбщЃК

ЂйаДГіЩњГЩAЕФРызгЗНГЬЪНЃК_________ЁЃ
ЂкЩЯЪіСїГЬжаЃЌМгШызуСПЯЁЯѕЫсЕФФПЕФЪЧ________ЁЃШчКЮХаЖЈЮоЩЋШмвКBгыЯЁЯѕЫсЗЂЩњСЫРызгЗДгІЃП________ЁЃ
ВщПДД№АИКЭНтЮі>>
ПЦФПЃКИпжаЛЏбЇ РДдДЃК ЬтаЭЃК
ЁОЬтФПЁПЯжгавдЯТМИжжгаЛњЮяЃК
Ђй![]() Ђк
Ђк![]() Ђл
Ђл Ђм
Ђм![]() Ђн
Ђн![]()
Ђо![]() Ђп
Ђп Ђр
Ђр![]() Ђс
Ђс![]()
ЧыРћгУЩЯЪіИјГіЕФЮяжЪАДвЊЧѓЛиД№ЯТСаЮЪЬтЃК
ЃЈ1ЃЉЂлЕФЯЕЭГУќУћЪЧ________ЁЃ
ЃЈ2ЃЉгУЁАЃОЁББэЪОЂйЂлЂмЂрШлЗаЕуИпЕЭЫГађЃК________ЃЈЬюађКХЃЉЁЃ
ЃЈ3ЃЉгыЂлЛЅЮЊЭЌЯЕЮяЕФЪЧ________ЃЈЬюађКХЃЉЁЃ
ЃЈ4ЃЉЂсЕФвЛТШДњЮяЭЌЗжвьЙЙЬхЪ§ФПга________жжЁЃ
ЃЈ5ЃЉдк120ЁцЃЌ![]() ЬѕМўЯТЃЌФГжжЦјЬЌЬўгызуСПЕФ
ЬѕМўЯТЃЌФГжжЦјЬЌЬўгызуСПЕФ![]() ЭъШЋЗДгІКѓЃЌВтЕУЗДгІЧАКѓЦјЬхЕФЬхЛ§УЛгаЗЂЩњИФБфЃЌдђИУЬўЪЧ________ЃЈЬюађКХЃЉЁЃ
ЭъШЋЗДгІКѓЃЌВтЕУЗДгІЧАКѓЦјЬхЕФЬхЛ§УЛгаЗЂЩњИФБфЃЌдђИУЬўЪЧ________ЃЈЬюађКХЃЉЁЃ
ЃЈ6ЃЉаДГіЂодкЬњзїДпЛЏМСЕФЬѕМўЯТгывКфхЗЂЩњШЁДњЗДгІЕФЛЏбЇЗНГЬЪН________ЁЃ
ВщПДД№АИКЭНтЮі>>
ПЦФПЃКИпжаЛЏбЇ РДдДЃК ЬтаЭЃК
ЁОЬтФПЁПЯТСаЪЕбщВйзїФмДяЕНЪЕбщФПЕФЕФЪЧ
A. гУОЫЎЪЊШѓЕФpHЪджНВтСПШмвКЕФpH
B. НЋ4.0 g NaOHЙЬЬхжУгк100 mLШнСПЦПжаЃЌМгЫЎжСПЬЖШЃЌХфжЦ1.000 molЁЄL1NaOHШмвК
C. гУзАжУМзеєИЩAlCl3ШмвКжЦЮоЫЎAlCl3ЙЬЬх
D. гУзАжУввГ§ШЅЪЕбщЪвЫљжЦввЯЉжаЕФЩйСПSO2
ВщПДД№АИКЭНтЮі>>
ПЦФПЃКИпжаЛЏбЇ РДдДЃК ЬтаЭЃК
ЁОЬтФПЁПЩш NAЮЊАЂЗќМгЕТТоГЃЪ§ЕФжЕЃЌЯТСаЫЕЗЈе§ШЗЕФЪЧ( )
A.зуСПЭгы 100mL 18mol/L ЕФХЈСђЫсдквЛЖЈЬѕМўЯТЗДгІзЊвЦЕФЕчзгЪ§ФПЮЊ1.8 NA
B.ГЃЮТЯТЃЌpH=13 ЕФЧтбѕЛЏФЦШмвКжагЩЫЎЕчРыГіЕФH+ЕФЪ§ФПЮЊ 10-13NA
C.БъзМзДПіЯТЃЌ2.24 L CCl4КЌгаЕФЙВМлМќЪ§ЮЊ 0.4NA
D.МгШШЬѕМўЯТЃЌ16g O2ЁЂO3ЕФЛьКЯЦјЬхгызуСПУОЗлГфЗжЗДгІзЊвЦЕФЕчзгЪ§ФПЮЊ 2NA
ВщПДД№АИКЭНтЮі>>
ПЦФПЃКИпжаЛЏбЇ РДдДЃК ЬтаЭЃК
ЁОЬтФПЁПгУЭМЫљЪОзАжУМьбщввЯЉЪБВЛашвЊГ§дгЕФЪЧ
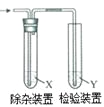
ввЯЉЕФжЦБИ | ЪдМСX | ЪдМСY | |
A | CH3CH2BrгыNaOHввДМШмвКЙВШШ | H2O | KMnO4ЫсадШмвК |
B | CH3CH2BrгыNaOHввДМШмвКЙВШШ | H2O | Br2ЕФCCl4ШмвК |
C | CH3CH2OHгыХЈH2SO4ЙВШШжС170Ёц | NaOHШмвК | KMnO4ЫсадШмвК |
D | CH3CH2OHгыХЈH2SO4ЙВШШжС170Ёц | NaOHШмвК | Br2ЕФCCl4ШмвК |
ВщПДД№АИКЭНтЮі>>
ПЦФПЃКИпжаЛЏбЇ РДдДЃК ЬтаЭЃК
ЁОЬтФПЁПЪвЮТЯТЃЌЯђФГNa2CO3КЭNaHCO3ЕФЛьКЯШмвКжаж№ЕЮМгШыBaCl2ШмвКЃЌШмвКжаlgc(Ba2+)гы![]() ЕФБфЛЏЙиЯЕШчЭМЫљЪОЁЃЯТСаЫЕЗЈе§ШЗЕФЪЧЃЈ ЃЉ
ЕФБфЛЏЙиЯЕШчЭМЫљЪОЁЃЯТСаЫЕЗЈе§ШЗЕФЪЧЃЈ ЃЉ
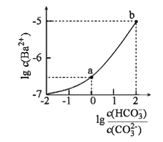
(вбжЊЃКH2CO3ЕФKa1ЁЂKa2ЗжБ№ЮЊ4.2ЁС10-7ЁЂ5.6ЁС10-11)
A.aЖдгІШмвКЕФpHаЁгкb
B.bЖдгІШмвКЕФc(H+)=4.2ЁС10-7molЁЄL-1
C.aЁњbЖдгІЕФШмвКжа![]() МѕаЁ
МѕаЁ
D.aЖдгІЕФШмвКжавЛЖЈДцдкЃК2c(Ba2+)+c(Na+)+c(H+)=3c(HCO3-)+c(Cl-)+c(OH-)
ВщПДД№АИКЭНтЮі>>
АйЖШжТаХ - СЗЯАВсСаБэ - ЪдЬтСаБэ
КўББЪЁЛЅСЊЭјЮЅЗЈКЭВЛСМаХЯЂОйБЈЦНЬЈ | ЭјЩЯгаКІаХЯЂОйБЈзЈЧј | ЕчаХеЉЦОйБЈзЈЧј | ЩцРњЪЗащЮожївхгаКІаХЯЂОйБЈзЈЧј | ЩцЦѓЧжШЈОйБЈзЈЧј
ЮЅЗЈКЭВЛСМаХЯЂОйБЈЕчЛАЃК027-86699610 ОйБЈгЪЯфЃК58377363@163.com