
����ʵ�鷽���У����ܴﵽʵ��Ŀ�ĵ���
ѡ�� | ʵ��Ŀ�� | ʵ�鷽�� |
A | ����CH3CH2Br��NaOH��Һ���Ƿ���ˮ�� | ��CH3CH2Br��NaOH��Һ���ȡ���ȴ��ȡ���ϲ�ˮ��Һ����ϡHNO3�ữ������AgNO3��Һ���۲��Ƿ��������ɫ���� |
B | ����Fe(NO3)2�����Ƿ����������� | ��Fe(NO3)2��Ʒ����ϡH2SO4�μ�KSCN��Һ���۲���Һ�Ƿ��� |
C | ��֤Br2��������ǿ��I2 | ��������ˮ����KI��Һ�У��ټ���CCl4�������ã��ɹ۲쵽�²�Һ�����ɫ |
D | ��֤AgI���ܽ��С��AgCl | ��NaIŨ��Һ����AgCl����Һ�У����ɹ۲쵽�����ɰ�ɫ��Ϊ��ɫ |
A. A B. B C. C D. D

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ��2017�����ʡ�����и�����ѧ�ڵ�������Ӧ�Կ������ۻ�ѧ�Ծ��������棩 ���ͣ�ѡ����
��11.2gͭ�ۺ�þ�۵Ļ����ֳ����ȷݣ�����һ�ݼ���200mL��ϡ�����в����ȣ����������ǡ����ȫ��Ӧ����������״���µ�NO2.24L������һ���ڿ����г�ּ��ȣ����õ�mg���塣�����й�˵������c(HNO3)=2mol��L-l��c(HNO3)=0.5mol��L-1��m=8.0��m=7.2������ȷ���ǣ� ��
A���٢� B���٢� C���ڢ� D���ڢ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2016-2017ѧ�����ʡ�߶�3���¿���ѧ�Ծ��������棩 ���ͣ��ƶ���
�ϳɹ�̽���һ��·�����£�
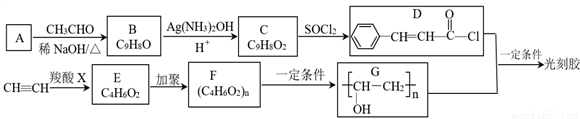

��RCOOH+CH CH��RCOOCH=CH2
CH��RCOOCH=CH2
�ش���������
��1����̽������������ŵ�������______��
��2��D+G����̽��Ļ�ѧ����ʽΪ______���÷�Ӧ�ķ�Ӧ������______��
��3��H��C��ͬ���칹�壬H�����������ʻ����������ܷ���ˮ�ⷴӦ��������Ӧ������ʹ��ˮ��ɫ�������ڷ����廯�����H�Ľṹ��______�֡����к˴Ź�������Ϊ5��壬�ҷ������Ϊ1��1��2��2��2�Ľṹ��ʽΪ______��
��4����������֪ʶ����������Ϣ��д����CH3CH2OHΪԭ���Ʊ�CH3CH2CH2COOC2H5�ĺϳ�·������ͼ(���Լ���ѡ)��
���ϳ�·������ͼʾ�����£� ��
��
______
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2016-2017ѧ�����ʡ�߶�3���¿���ѧ�Ծ��������棩 ���ͣ�ѡ����
��һ�������£���ӦA2 + B2  2AB ��H= Q kJ/mol�ķ�Ӧʱ����������AB������������AB%���Ĺ�ϵ��ͼ��ʾ������a��bΪ��ͬ�¶�ʱ�ķ�Ӧ���ߣ������������䣩��cΪ��t3ʱ�̿�ʼ��С�����ݻ����¶Ȳ��䣩�ı仯���ߡ������й�˵����ȷ����
2AB ��H= Q kJ/mol�ķ�Ӧʱ����������AB������������AB%���Ĺ�ϵ��ͼ��ʾ������a��bΪ��ͬ�¶�ʱ�ķ�Ӧ���ߣ������������䣩��cΪ��t3ʱ�̿�ʼ��С�����ݻ����¶Ȳ��䣩�ı仯���ߡ������й�˵����ȷ����
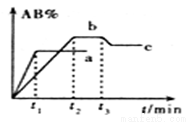
A. Q >0
B. A2��B2��������һ������̬����
C. b��Ӧ��ƽ��״̬ʱ��ѧ��Ӧ���ʱ�c��Ӧ��ƽ��״̬ʱ��ѧ��Ӧ���ʴ�
D. ABһ������������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2017������и�����ѧ�ڵ�һ���¿���ѧ�Ծ��������棩 ���ͣ��ƶ���
�л���M���л��ϳɵ���Ҫ�м��壬�Ʊ�M��һ�ֺϳ�·�����£����ַ�Ӧ�������Լ�����ȥ��
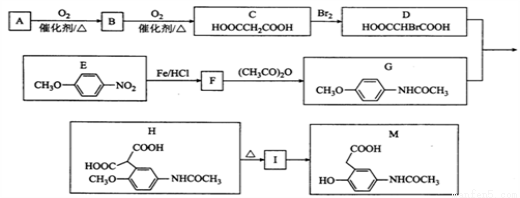
��֪�� ��A���ܶ�����ͬ������H2�ܶȵ�38��������ӵĺ˴Ź�����������3��壻
��
��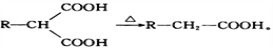
��ش��������⣺
��1��B�Ļ�ѧ����Ϊ______________��A�й����ŵĵ���ʽΪ________________��
��2��C�й����ԭ�������________����I�Ľṹ��ʽΪ_____________________��
��3��F��G�Ļ�ѧ����ʽΪ________________________________________________��
��4��M�����ܷ����ķ�ӦΪ_______________����ѡ����ĸ��
A.�ӳɷ�Ӧ B.������Ӧ C.ȡ����Ӧ D.��ȥ��Ӧ
��5��ͬʱ��������������E��ͬ���칹����_________�֡�
������FeCl3��Һ������ɫ��Ӧ ������NaHCO3��Ӧ �ۺ��С�����NH2
��6�����������ϳ�·�ߣ��� 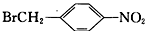 Ϊԭ�ϣ����Լ���ѡ��������Ʊ�
Ϊԭ�ϣ����Լ���ѡ��������Ʊ�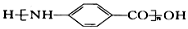 �ĺϳ�·�ߣ�_______________________________________________��
�ĺϳ�·�ߣ�_______________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2016-2017ѧ��ɽ��ʡ��һ3���¿���ѧ�Ծ��������棩 ���ͣ������
��֪A��B��C��D��E�������ڱ���ǰ�����ڵ�Ԫ�أ����ǵĺ˵����A<B<C<D<E������A��B��C��ͬһ���ڵķǽ���Ԫ�ء�������DCΪ���ӻ����D�Ķ�����������C�������Ӿ�����ͬ�ĵ��Ӳ�ṹ��AC2Ϊ�Ǽ��Է���,�Dz�������ЧӦ����Ҫ���塣B��C���⻯��ķе������ͬ����������Ԫ���⻯��ķе�ߡ�����ȱEԪ�ػ�����Dz���D��Eλ��ͬ���塣���������������ش��������⣺(����ʱ��A��B��C��D��E������Ӧ��Ԫ�ط��ű�ʾ)
(1)A��B��C�ǽ�������ǿ������˳��Ϊ________��
(2)B���⻯��ķ���ʽ��________��B���⻯������ˮ�ĵ��뷽��ʽΪ
________________________________________________________________________��
(3)д��������AC2�ĵ���ʽ��________������________(����ԡ��Ǽ��ԡ�)���γɵķǼ��Է��ӡ�
(4)B������������Ӧ��ˮ�����ϡ��Һ��D�ĵ��ʷ�Ӧʱ��B����ԭ����ͼۣ��÷�Ӧ�Ļ�ѧ����ʽ��_________________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2016-2017ѧ��ɽ��ʡ��һ3���¿���ѧ�Ծ��������棩 ���ͣ�ѡ����
��ͼ��Ԫ�����ڱ���һ����,��֪������Ӱ�м��3������ͬһ�塣�й���Ӱ���ֵ�Ԫ��,����˵����ȷ����(����)

A. ��������Ԫ�� B. ���Ǹ���Ԫ��
C. ��5�ָ���Ԫ�غ�2������Ԫ�� D. ��5������Ԫ�غ�2�ָ���Ԫ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ʡ���IJ���ѧ2017�������ѧ��������ѧ�Ծ� ���ͣ������
������(CH3OCH3)����Ϊ21���͵�����ȼ�ϣ���CO��H2Ϊԭ��������������Ҫ��������������Ӧ��
��ѧ��Ӧ����ʽ | ��ѧƽ�ⳣ�� | |
��CO(g)��2H2(g) | ��H1=-99 kJ•mol-1 | K1 |
��2CH3OH(g) | ��H2����24 kJ•mol-1 | K2 |
��CO(g)��H2O(g) | ��H3����41 kJ•mol-1 | K3 |
��1���ù��յ��ܷ�ӦΪ3CO(g)��3H2(g) CH3OCH3(g)��CO2(g) ��H
CH3OCH3(g)��CO2(g) ��H
�÷�Ӧ��H��__________________����ѧƽ�ⳣ��K��____________________(�ú�K1��K2��K3�Ĵ���ʽ��ʾ)��
��2��ij�¶��£���8.0molH2��4.0molCO�����ݻ�Ϊ2L���ܱ������У�������Ӧ��4H2(g)+2CO(g)  CH3OCH3(g)+H2O(g)��10 ���Ӻ�Ӧ��ƽ�⣬��ö����ѵ��������Ϊ25%����CO��ת����Ϊ________��
CH3OCH3(g)+H2O(g)��10 ���Ӻ�Ӧ��ƽ�⣬��ö����ѵ��������Ϊ25%����CO��ת����Ϊ________��
��3�����д�ʩ�У������CH3OCH3���ʵ���________��
A������������� B�������¶� C�����ø�Ч���� D������ѹǿ
��4���ù����з�Ӧ�۵ķ��������CH3OCH3�IJ��ʣ�ԭ����_______________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2016-2017ѧ���Ĵ�ʡ�ɶ��б���У���߶�3���¿���ѧ�Ծ��������棩 ���ͣ�ѡ����
��NO3����Ϊ�ȵ�������ǣ� ��
A. SO32- B. BF3 C. CH4 D. NO2
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com