
 ���������仯�����ںϽ�����Լ������ȷ���Ӧ�ù㷺������Ҫ��ش��������⣮
���������仯�����ںϽ�����Լ������ȷ���Ӧ�ù㷺������Ҫ��ش��������⣮ �����������Ⱥ�ɫ��������A��
�����������Ⱥ�ɫ��������A������ ��1��Ni��28��Ԫ�أ������������ԭ������������ԭ���ͺ��ع���д�����������Ų�ʽΪ��1s22s22p43s23p43d84s2���ݴ��ж���۵����Ų�ʽ��
��2�����ݷ��Ӿ�����۷е�ϵͷ�����
��3����ͬһ�����У�Ԫ�صĵ縺������ԭ������������������ݼ۲���ӶԻ�������ȷ������ԭ���ӻ���ʽ��C-CΪ̼̼�Ҽ���C=N����һ��̼���Ҽ���
��Ni2+���пչ����Nԭ�Ӻ��й¶Ե��Ӷԣ�Nԭ����Ni2+�γ���λ������ԭ������ԭ��֮���γ������
��4�����Ӿ�����۵������Ӽ���ǿ���йأ��������ӵ����ͬ�����Ӱ뾶Խ������ԽС���۵�Խ�ͣ�ͬ�־���ṹ���͵���λ����ͬ��
��5�����þ�̯��ȷ�������Ļ�ѧʽ��
��� �⣺��1��Ni��28��Ԫ�أ����������Ų�ʽΪ��1s22s22p43s23p43d84s2���۵�����Ϊ10���۵����Ų�ʽΪ3d84s2��
�ʴ�Ϊ��3d84s2��
��2��Ni��CO��4����ΪҺ̬��������CCl4�������л��ܼ�����Ni��CO��4���ڷ��Ӿ��壻
�ʴ�Ϊ�����ӣ�
��3����ͬһ�����У�Ԫ�صĵ縺������ԭ�������������������ĵ縺����С������C��N��O��H����Ԫ�صĵ縺�Դ�С˳��Ϊ��O��N��C��H������̼ԭ�Ӽ۲���ӶԸ�����4�Ҳ����µ��Ӷԣ�Ϊsp3�ӻ������Ӽ���̼ԭ�Ӻ���3���۲���Ӷ��Ҳ����µ��Ӷԣ�Ϊsp2�ӻ���C-CΪ̼̼�Ҽ���C=N����һ��̼���Ҽ�����1mol�÷����к��е�̼̼�Ҽ���̼���Ҽ�������Ϊ5NA��
�ʴ�Ϊ��O��N��C��H��sp3��sp2��5NA��
��Ni2+���пչ����Nԭ�Ӻ��й¶Ե��Ӷԣ�Nԭ����Ni2+�γ���λ������ͬ��������ԭ������ԭ��֮���γ��������ͼ��ʾ��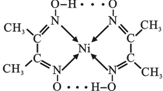 ��
��
�ʴ�Ϊ�� ��
��
��4��NiO��FeO�ľ���ṹ���;����Ȼ��Ƶ���ͬ��˵�����߶������Ӿ��壬�������������Խ�࣬���Ӱ뾶ԽС��������Խ���۵�Խ�ߣ�����Ni2+�����Ӱ뾶С��Fe2+�����Ӱ뾶�������۵��ǣ�FeO��NiO����ΪNi0��Fe0�ľ���ṹ���;����Ȼ��Ƶ���ͬ�����Ȼ������������ӵ���λ����Ϊ6������Ni0������Ni��O����λ��Ҳ��Ϊ6��
�ʴ�Ϊ������6��
��5��̼ԭ��λ�ڸþ����������ϣ����Ըþ����к���һ��̼ԭ�ӣ�þԭ�Ӹ���=$\frac{1}{8}$��8�����Ըþ�������1��þԭ�ӣ���ԭ�Ӹ���=$\frac{1}{2}$��6���þ����к���3����ԭ�ӣ����Ըþ����Ļ�ѧʽΪMgNi3C��
�ʴ�Ϊ��MgNi3C��
���� ���⿼���˼۵��ӵ��Ų�ʽ����λ����ԭ���ӻ���ʽ�жϡ������۷е�ߵͱȽϵ�֪ʶ�㣬�ѵ�����λ����ʾ��������ȷ��λ�������ṩ�µ��ӶԵ�ԭ��ָ���ṩ�չ����ԭ���ǽ��ؼ�����Ŀ�Ѷ��еȣ�



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ���������ʱ�����ɵ������������������ӵ��Ǽ� | |
| B�� | ij�����������״̬�ܵ��磬�û�������һ�������Ӽ� | |
| C�� | ij�������ˮ��Һ�ܵ��磬�û�����һ���ǵ���� | |
| D�� | ij�������ڳ�����Ϊ���壬����ɸ����ʵ���һ�����й��ۼ� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ����Ͳ��ȡ20.21mL��������Ϊ98%��ŨH2SO4 | |
| B�� | ��5.85g NaCl����100mLˮ�У��Ƶ�1 mol/LNaCl��Һ | |
| C�� | ��22.4LHCl��������ˮ���1 L��Ũ��Ϊ1 mol/L������ | |
| D�� | ��1���cmol/L��������ˮϡ��Ϊ5������Ի��Ũ��Ϊ0.2cmol/L������ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ��ҵ����������������FeS2��Ϊԭ���Ʊ����� | |
| B�� | ���NaCl��Һ�Ʊ�������ͬʱ�õ�Cl2 | |
| C�� | ��H2��CO��ԭAl2O3�Ʊ������� | |
| D�� | ���ѻ��ķ�������������ϩ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | �������֬����������������Һ��Ӧ | |
| B�� | �����Ҵ����Ƿ���ˮ�ɼ���������ˮ����ͭ��������ɫ��ˮ | |
| C�� | �ڵ�����Һ�м���20%��ϡ����ˮ���������������Cu��OH��2����Һ���ȣ�֤�������������� | |
| D�� | �ڼ�������Һ�м��루NH4��2SO4������Һ���г����������ټ�������ˮ�������ܽ� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ����������һ�����õ�ɱ������Ҳ��������������ˮ | |
| B�� | ���ڼ��õ����ȼ����Ҫ�����ࣺһ����ѹ����Ȼ������һ��ΪҺ��ʯ���������Ƕ������л��� | |
| C�� | Ϊ�����øϴ�·۵�ϴ��Ч���������ȵ�����ˮ�ܽ�ϴ�·� | |
| D�� | ���������������ļӿ죬�����С��װʳƷ�ѱ��㷺���ܣ�Ϊ�˷�ֹ�����±��ȸ�֬ʳƷ�������ʣ��ӳ�ʳƷ�ı����ڣ���ȷ���������ڰ�װ���г�������ʯ�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ���ѡ��

| A�� | n=0.02 | |
| B�� | y=2240 | |
| C�� | ԭ�������FeS04����������ԼΪ89% | |
| D�� | m=3.2 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
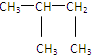 ��
��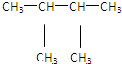 ⑪${\;}_{17}^{35}$Cl⑫
⑪${\;}_{17}^{35}$Cl⑫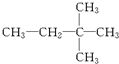
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com